Abstract
Background:
Development of valvular substitutes meeting the performance criteria for surgical correction of congenital heart malformations is a major research challenge. The sheep is probably the most widely used animal model in heart valves regenerative medicine. Although the standard cardiopulmonary bypass (CPB) technique and various anesthetic and surgical protocols are reported to be feasible and safe, they are associated with significant morbidity and mortality rates. The premise of this paper is that the surgical technique itself, especially the perioperative animal care and management protocol, is essential for successful outcomes and survival.
Methods:
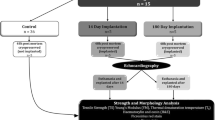
Ten juvenile and adult female sheep aged 7.8–37.5 months and weighing 32.0–58.0 kg underwent orthotopic implantation of tissue-engineered pulmonary valve conduits on beating heart under normothermic CPB. The animals were followed-up for 6 months before scheduled euthanasia.
Results:
Based on our observations, we established a guide for perioperative care, follow-up, and treatment containing information regarding the appropriate clinical, biological, and ultrasound examinations and recommendations for feasible and safe anesthetic, surgical, and euthanasia protocols. Specific recommendations were also included for perioperative care of juvenile versus adult sheep.
Conclusion:
The described surgical technique was feasible, with a low mortality rate and minimal surgical complications. The proposed anesthetic protocol was safe and effective, ensuring both adequate sedation and analgesia as well as rapid recovery from anesthesia without significant complications. The established guide for postoperative care, follow-up and treatment in sheep after open-heart surgery may help other research teams working in the field of heart valves tissue regeneration.










Similar content being viewed by others
References
Salerno CT, Droel J, Bianco RW. Current state of in vivo preclinical heart valve evaluation. J Heart Valve Dis. 1998;7:158–62.
Choo SJ, Kim KI, Park NH, Song JM, Choi IC, Shim JY, et al. Development of an animal experimental model for a bileaflet mechanical heart valve prosthesis. J Korean Med Sci. 2004;19:37–41.
DiVincenti L Jr, Westcott R, Lee C. Sheep (Ovis aries) as a model for cardiovascular surgery and management before, during, and after cardiopulmonary bypass. J Am Assoc Lab Anim Sci. 2014;53:439–48.
Leroux AA, Moonen ML, Pierard LA, Kolh P, Amory H. Animal models of mitral regurgitation induced by mitral valve chordae tendineae rupture. J Heart Valve Dis. 2012;21:416–23.
Harpa MM, Movileanu I, Sierad LN, Cotoi SO, Suciu H, Sircuta C, et al. Pulmonary heart valve replacement using stabilized acellular xenogeneic scaffolds; effects of seeding with autologous stem cells. Rev Rom Med Lab. 2015;23:415–30.
Ionela M, Klara B, Marius H, Dan N, Ovidiu C, Preda T, et al. Pressurized perfusion system for obtaining completely acellular pulmonary valve scaffolds for tissue engineering. ARS Medica Tomitana. 2019;25:149–56.
Valverde A, Doherty JT. Anesthesia and analgesia of ruminants. In: Fish ER, Brown JM, Danneman JP, Karas ZA, editors. Anesthesia and analgesia in laboratory animals. 2nd ed. London: Elsevier; 2008. p. 406–7.
Jackson GGP, Cockroft DP. Clinical examination of farm animals. Oxford: Blackwell Science; 2002.
Sousa RS, Minervino AH, Araújo CA, Rodrigues FA, Oliveira FL, Mori CS, et al. Clinical response and transfusion reactions of sheep subjected to single homologous blood transfusion. ScientificWorldJournal. 2014;2014:734397.
Souza HJ, Palma JH, Casagrande IS, Christo SC, Alves-Silva LS, Almeida MA, et al. Replacement of pulmonary artery trunk in sheep using tubular valved heterograft in non-aldehydic preservation. Rev Bras Cir Cardiovasc. 2012;27:419–28.
Flameng W, Jashari R, De Visscher G, Mesure L, Meuris B. Calcification of allograft and stentless xenograft valves for right ventricular outflow tract reconstruction: an experimental study in adolescent sheep. J Thorac Cardiovasc Surg. 2011;141:1513–21.
Mendelson K, Schoen FJ. Heart valve tissue engineering: concepts, approaches, progress, and challenges. Ann Biomed Eng. 2006;34:1799–819.
Heard AM, Green RJ, Eakins P. The formulation and introduction of a ’can’t intubate, can’t ventilate’ algorithm into clinical practice. Anaesthesia. 2009;64:601–8.
Schweiger M, Knirsch W, Cesarovic N, Krüger B, Schmiady M, Frauenfelder T, et al. Surgical technique: establishing a pre-clinical large animal model to test aortic valve leaflet substitute. J Thorac Dis. 2016;8:3733–8.
Ashworth A, Klein AA. Cell salvage as part of a blood conservation strategy in anaesthesia. Br J Anaesth. 2010;105:401–16.
Kellett-Gregory LM, Seth M, Adamantos S, Chan DL. Autologous canine red blood cell transfusion using cell salvage devices. J Vet Emerg Crit Care (San Antonio). 2013;23:82–6.
Smith CJ, Danneman JP. Monitoring of anesthesia. In: Fish ER, Brown JM, Danneman JP, Karas ZA, editors. Anesthesia and analgesia in laboratory animals. 2nd ed. London: Elsevier; 2008. p. 176–7.
England GC, Clarke KW. Alpha 2 adrenoceptor agonists in the horse—a review. Br Vet J. 1996;152:641–57.
Al Hussein H, Harpa M, Movileanu I, Al Hussein H, Suciu H, Branzaniuc K, et al. Minimally invasive surgical protocol for adipose derived stem cells collection and isolation—ovine model. Rev Chim. 2019;70:1826–8.
Abrahamsen EJ. Chemical restraint and injectable anesthesia of ruminants. Vet Clin North Am Food Anim Pract. 2013;29:209–27.
Phillips W, Anderson A, Rosengreen M, Johnson J, Halpin J. Propofol versus propofol/ketamine for brief painful procedures in the emergency department: clinical and bispectral index scale comparison. J Pain Palliat Care Pharmacother. 2010;24:349–55.
Miner JR, Burton JH. Clinical practice advisory: emergency department procedural sedation with propofol. Ann Emerg Med. 2007;50:182–7.
Shah A, Mosdossy G, McLeod S, Lehnhardt K, Peddle M, Rieder M. A blinded, randomized controlled trial to evaluate ketamine/propofol versus ketamine alone for procedural sedation in children. Ann Emerg Med. 2011;57:425–33.e2.
Andolfatto G, Willman E. A prospective case series of pediatric procedural sedation and analgesia in the emergency department using single-syringe ketamine-propofol combination (ketofol). Acad Emerg Med. 2010;17:194–201.
Willman EV, Andolfatto G. A prospective evaluation of “ketofol” (ketamine/propofol combination) for procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2007;49:23–30.
Lin HC, Purohit RC, Powe TA. Anesthesia in sheep with propofol or with xylazine-ketamine followed by halothane. Vet Surg. 1997;26:247–52.
Knirsch W, Krüger B, Fleischmann T, Malbon A, Lipiski M, Lemme F, et al. Establishing a pre-clinical growing animal model to test a tissue engineered valved pulmonary conduit. J Thorac Dis. 2020;12:1070–8.
Harper NJN, Cook TM, Garcez T, Lucas DN, Thomas M, Kemp H, et al. Anaesthesia, surgery, and life-threatening allergic reactions: management and outcomes in the 6th National Audit Project (NAP6). Br J Anaesth. 2018;121:172–88.
Prassinos NN, Galatos AD, Raptopoulos D. A comparison of propofol, thiopental or ketamine as induction agents in goats. Vet Anaesth Analg. 2005;32:289–96.
Green AS. Veterinary anesthesia and pain management secrets. Philadelphia: Hanley & Belfus; 2002.
Hikasaa Y, Saitob K, Takaseb K, Ogasawarab S. Clinical, cardiopulmonary, hematological and serum biochemical effects of sevoflurane and isoflurane anesthesia in oxygen under spontaneous breathing in sheep. Small Rumin Res. 2000;36:241–9.
Davidson GS. Equine anesthesia: triple drip. Int J Pharm Compd. 2008;12:402–4.
Ali ML, Kumar SP, Bjornstad K, Duran CM. The sheep as an animal model for heart valve research. Cardiovasc Surg. 1996;4:543–9.
Finley A, Greenberg C. Review article: heparin sensitivity and resistance: management during cardiopulmonary bypass. Anesth Analg. 2013;116:1210–22.
Carr JA, Silverman N. The heparin-protamine interaction. A review. J Cardiovasc Surg (Torino). 1999;40:659–66.
Hoenicka M, Rupp P, Müller-Eising K, Deininger S, Kunert A, Liebold A, et al. Anticoagulation management during multivessel coronary artery bypass grafting: a randomized trial comparing individualized heparin management and conventional hemostasis management. J Thromb Haemost. 2015;13:1196–206.
Boer C, Meesters MI, Veerhoek D, Vonk ABA. Anticoagulant and side-effects of protamine in cardiac surgery: a narrative review. Br J Anaesth. 2018;120:914–27.
Carney EL, Clark JB, Myers JL, Peterson R, Wilson RP, Weiss WJ. Animal model development for the Penn State pediatric ventricular assist device. Artif Organs. 2009;33:953–7.
Shofti R, Zaretzki A, Cohen E, Engel A, Bar-El Y. The sheep as a model for coronary artery bypass surgery. Lab Anim. 2004;38:149–57.
Murphy GS, Hessel EA 2nd, Groom RC. Optimal perfusion during cardiopulmonary bypass: an evidence-based approach. Anesth Analg. 2009;108:1394–417.
Magruder JT, Crawford TC, Harness HL, Grimm JC, Suarez-Pierre A, Wierschke C, et al. A pilot goal-directed perfusion initiative is associated with less acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg. 2017;153:118–25.e1.
Ranucci M, Romitti F, Isgrò G, Cotza M, Brozzi S, Boncilli A, et al. Oxygen delivery during cardiopulmonary bypass and acute renal failure after coronary operations. Ann Thorac Surg. 2005;80:2213–20.
Magruder JT, Dungan SP, Grimm JC, Harness HL, Wierschke C, Castillejo S, et al. Nadir oxygen delivery on bypass and hypotension increase acute kidney injury risk after cardiac operations. Ann Thorac Surg. 2015;100:1697–703.
Levy JH, Tanaka KA. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 2003;75:S715–20.
Manrique AM, Vargas PD, Palmer D, Kelly K, Litchenstein SE. The effects of cardiopulmonary bypass following pediatric cardiac surgery. In: Munoz R, Morell V, da Cruz E, Vetterly C, da Silva J, editors. Critical care of children with heart disease. Cham: Springer; 2020. p. 113–29.
Luciani GB, Menon T, Vecchi B, Auriemma S, Mazzucco A. Modified ultrafiltration reduces morbidity after adult cardiac operations: a prospective, randomized clinical trial. Circulation. 2001;104:I253–9.
Torina AG, Petrucci O, Oliveira PP, Severino ES, Vilarinho KA, Lavagnoli C, et al. The effects of modified ultrafiltration on pulmonary function and transfusion requirements in patients underwent coronary artery bypass graft surgery. Rev Bras Cir Cardiovasc. 2010;25:59–65.
Naveed A, Azam H, Murtaza HG, Ahmad RA, Baig MAR. Incidence and risk factors of pulmonary complications after cardiopulmonary bypass. Pak J Med Sci. 2017;33:993–6.
Lako S, Dedej T, Nurka T, Ostreni V, Demiraj A, Xhaxho R, et al. Hematological changes in patients undergoing coronary artery bypass surgery: a prospective study. Med Arch. 2015;69:181–6.
Acknowledgments
This work was supported by a grant from the Competitiveness Operational Programme 2014-2020, Tissue engineering technologies for cardiac valve regeneration, valve-regen, ID:P_37_673, Mysmis code:103431, contract 50/05.09.2016, from grant 1P30GM131959 from NIGMS and from the Dempsey Endowment. We would like to thank Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no financial conflicts of interest.
Ethical statement
All procedures and perioperative care of the experimental animals were performed in accordance with the “Guide for the care and use of laboratory animals” and Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. Euthanasia was performed in compliance with the Convention on the experiments on live vertebrate animals and all the legal norms referring to the protection and welfare of animals: Law No. 9 of January 11, 2008 on the animals’ protection and Decision no. 19 of 01. July 2011 adopted by the National Council of the Veterinary Doctors College regarding the “Guide for the euthanasia of animals.” This work is part of a research grant conducted in accordance with the protocol no. 131/21.10.2016 approved by the Ethics Committee of the UMFST “George Emil Palade” of Tirgu Mures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al Hussein, H., Al Hussein, H., Sircuta, C. et al. Challenges in Perioperative Animal Care for Orthotopic Implantation of Tissue-Engineered Pulmonary Valves in the Ovine Model. Tissue Eng Regen Med 17, 847–862 (2020). https://doi.org/10.1007/s13770-020-00285-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-020-00285-1