Abstract
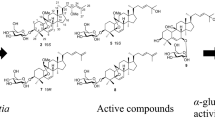
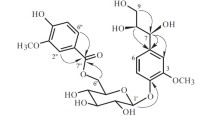
Ethyl acetate (EtOAc) layer obtained after the solvent fractionation of hot water extracts from nutgall tree (Rhus javanica) stem bark showed higher α-glucosidase inhibition activity than other layers. A novel acetophenone glucoside (4) and six known phenolic compounds were isolated from the EtOAc layer. The structure of 4 was determined to be 3,4,5-trihydroxyacetophenone 4-O-β-d-glucopyranoside. The six known compounds were identified as gallic acid (1), 5-methylresorcinol (2), methylgallate (3), 3-hydroxy-5-methylphenol 1-O-β-d-(6′-galloyl)glucopyranoside (5), scopoletin (6), and phlorizin (7). Their chemical structures were determined by electrospray ionization mass spectrometry and nuclear magnetic resonance analyses. Compound 5 was newly identified from this plant. Compounds 6 and 7 showed significantly higher α-glucosidase inhibition activity than other compounds.
Similar content being viewed by others
References
Benalla W, Bellahcen S, and Bnouham M (2010) Antidiabetic medicinal plants as a source of alpha glucosidase inhibitors. Curr Diabetes Rev 6, 247–254.
Cho JY, Ji SH, Moon JH, Lee KH, Jung KH, and Park KH (2008) A novel benzoyl glucoside and phenolic compounds from the leaves of Camellia japonica. Food Sci Biotechnol 17, 1060–1065.
Chun CS, Kim JH, Lim HA, Sohn HY, Son KH, Kim YK et al. (2004) Antioxidative effect of Rhus javanica Linne extract against hydrogen peroxide or menadione induced oxidative stress and DNA damage in HepG2 cells. J Food Sci Nutr 9, 150–155.
Chung SC, Hwang BY, Oh GJ, Kang SJ, Kim MJ, Choi WH et al. (1999) Chemical components from the stem bark of Rhus javanica L. Kor J Pharmacogn 30, 295–300.
Goldstein BJ (2003) Insulin resistance: from benign to type 2 diabetes mellitus. Rev Cardiovasc Med 4,suppl 6, S3–S10.
Gu Q, Wang RR, Zhang XM, Wang YH, Zheng YT, Zhou J et al. (2007) A new benzofuranone and anti-HIV constituents from the stems of Rhus chinensis. Plant Med 73, 279–282.
Hanefeld M, Schaper F, and Koehler C (2008) Effect of acarbose on vascular disease in patients with abnormal glucose tolerance. Cardiovasc Drugs Ther 22, 225–231.
Islam MN, Jung HA, Sohn HS, Kim HM, and Choi JS (2013) Potent α-glucosidase and protein tyrosine phosphatase 1B inhibitors from Artemisia capillaris. Arch Pharm Res 35, 542–552.
Iwakawa T, Tanaka Y, and Takashima A (2001) A new galloylglucoside from Cleyera ochnacea DC. Chem Pharm Bull 49, 1498–1499.
Karaca M, Magnan C, and Kargar C (2009) Functional pancreatic beta-cell mass: Involvement in type 2 diabetes and therapeutic intervention. Diabetes & Metabolism 35, 77–84.
Kim SH, Park HH, Lee SY, Jun CD, Choi BJ, Kim SY et al. (2005) The antianaphylactic effect of the gallo of Rhus javanica is mediated through inhibition of histamine release and inflammatory cytokine secretion. Int Immunopharmacol 5, 1820–1829.
Kumar S, Narwal S, Kumar V, and Prakash O (2011) α-Glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharm Rev 5, 19–29.
Kurokawa M, Basnet P, Ohsugi M, Hozumi T, Kadota S, Namba T et al. (1999) Anti-herpes simplex virus activity of moronic acid purified from Rhus javanica in vitro and in vivo. J Pharm Exp Ther 289, 72–78.
Kwon EJ, Kim YC, Kwon MS, Kim CS, Kang WW, Lee JB et al. (2001) Antioxidative activity of solvent fraction and isolation of antioxidant compound from chestnut husk. J Korean Soc Food Sci Nutr 30, 726–731.
Lee IS, Oh SR, Ahn KS, and Lee HK. (2001) Semialactone, isofouquierone peroxide and fouquierone, three new dammarane triterpenes form Rhus javanica. Chem Pharm Bull 49, 1024–1026
Lee SK, Hwang JY, Song JH, Jo JR, Kim MJ, Kim ME et al. (2007) Inhibitory activity of Euonymus alatus against alpha-glucosidase in vitro and in vivo. Nutr Res Pract 1, 184–188.
Lin CN, Chen HL, and Yen MH (2008) Flavonoids with DNA strand-scission activity from Rhus javanica var. roxburghiana. Fitoterapia 79, 32–36.
Luo H, Imoto T, and Hiji Y (2001) Inhibitory effect of voglibose and gymnemic acid on maltose absorption in vivo. World J Gastroenterol 7, 270–274.
Najafian M, Jahromi MZ, Nowroznejhad MJ, Khajeaian P, Kargar MM, Sadeghi M et al. (2012) Phloridzin reduces blood glucose levels and improves lipids metabolism in streptozotocin-induced diabetic rats. Mol Biol Rep 39, 5299–5306.
Oh JY, Choi U, Kim YS, and Shin DH (2003) Isolation and identification of antioxidative components from bark of Rhus javanica Linne. Korean J Food Sci Technol 35, 726–732.
Reaven GM (1988) Role of insulin resistance in human disease. Diabetes 37, 1595–1607.
Rossetti L, Smith D, Shulman GI, Papachristou D, and DeFronzo RA (1987) Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest 79, 1510–1515.
Scheen AJ (2007) Antidiabetic agents in subjects with mild dysglycemia: prevent or early treatment of type 2 diabetes? Diabetes & Metabolism 33, 3–12.
Shim YJ, Doo HK, Ahn SY, Kim YS, Seong JK, Park IS et al. (2003) Inhibitory effect of aqueous extract from the gall of Rhus chinensis on alpha-glucosidase activity and postprandial blood glucose. J Ethnopharmacol 85, 35–37.
Standle E and Schnell O (2012) Alpha-glucosidase inhibitors 2012-Cardiovascular considerations and trial evaluation. Diab Vasc Dis Res 9, 163–169.
Wang RR, Gu Q, Wang YH, Zhang XM, Yang LM, Zhou J et al. (2008) Anti-HIV activities of compounds isolated from the medicinal plant Rhus chinensis. J Ethnopharmacol 117, 249–256.
Wansi JD, Lallemand MC, Chizem DD, Toze FAA, Mbaze LM, Naharkhan S et al. (2007) α-Glucosidase inhibitory constituents from stem bark of Terminalia superba (Combretaceae). Phytochemistry 68, 2096–2100.
Weihmeier UF and Piepersberg W (2004) Biotechnology and molecular biology of alpha-glucosidase inhibitor acarbose. Microbiol Biotechnol 63, 613–625.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cho, JY., Lee, KD., Park, SY. et al. Isolation and identification of α-glucosidase inhibitors from the stem bark of the nutgall tree (Rhus javanica Linné). J Korean Soc Appl Biol Chem 56, 547–552 (2013). https://doi.org/10.1007/s13765-013-3140-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13765-013-3140-7