Abstract
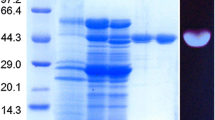
N-acetyl-β-d-hexosaminidase was purified from wheat bran and characterized. The purified enzyme showed two protein bands on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with molecular mass of 75 and 78 kDa. The enzyme exhibited optimum pH and temperature at 5.0 and 50°C, respectively. The enzyme was active on the substrates of p-nitrophenyl-N-acetyl-β-d-glucosaminide (pNP-GlcNAc) and p-nitrophenyl-N-acetyl-β-d-galactosaminide (pNP-GalNAc), whereas inactive on pNP-β-d-glucopyranoside, pNP-β-d-galactopyranoside, swollen chitin, and colloidal chitin, suggesting high substrate specificity. The enzyme activity for pNP-GlcNAc was stable at pH 3–6 and under 50°C. The K m , V max and K cat for pNP-GlcNAc were 0.014 mM, 0.05 μmol/min, and 3.01×106 min−1, respectively. The enzyme could be completely inhibited at 1–10 mM HgCl2 and AgNO3, suggesting that the intact thiol group is essential for activity. β-N-Acetylhexosaminidase from wheat bran could inhibit the conidial germination and digest the hyphae of Fusarium solani.
Similar content being viewed by others
References
Barber MS and Ride JP (1989) Purification and properties of a wheat leaf N-acetyl-β-d-hexosaminidase. Plant Sci 60, 163–172.
Bouquelet S and Spike G (1978) Properties of four molecular forms of N-acetyl-β-d-hexosaminidase isolated form germinating seeds of fenugreek (Trigonella foenum graecum). Eur J Biochem 84, 551–559.
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72, 248–254.
Brunner K, Peterbauer CK, Mach RL, Lorito M, Zeilinger S, and Kubicek CP (2003) The Nag1 N-acetylglucosaminidase of Trichoderma atroviride is essential for chitinase induction by chitin and of major relevance to biocontrol. Curr Genet 43, 289–290.
Chang CT, Young FP, Chang MH, and Sung HY (1998) Purification and properties of β-N-acetylhexosaminidase from cabbage. Biochem Mol Biol 45, 371–380.
Dey PM (1984) Occurrence of glycoprotein glycosidases in mature seeds of mung bean (Vigna radiate). Phytochem 23, 257–260.
Haran S, Schickler H, Oppenheim A, and Chet I (1995) New components of the chitinolytic system of Trichoderma harzianum. Mycol Res 99, 441–446.
Harley SM and Beevers L (1987) Isozymes of β-N-acetylhexosaminidase from pea seeds (Pisum sativum L.). Plant Physiol 85, 1118–1122.
Harris N and Chrispeels MJ (1975) Histochemical and biochemical observations on storage protein metabolism and protein body autolysis in cotyledons of germinating mung beans. Plant Physiol 56, 292–299.
Hart GW, Housley MP, and Slawson C (2007) Cycling of O-linked β-Nacetylglucosamine on nucleocytoplasmic proteins. Nature 446, 1017–1022.
Horsch M, Mayer C, Sennhauser U, and Rast DM (1997) β-NAcetylhexosaminidase: A target for the design of antifungal agents. Pharmacol Ther 76, 187–218.
Ishihara A, Miyagawa H, Matsukawa T, Ueno T, Mayama S, and Iwamura H (1998) Induction of hydroxyanthranilate hydroxycinnamoyl transferase activity by oligo-N-acetylchitooligosaccharides in oats. Phytochem 47, 969–974.
Jagadeesh BH, Prabha TN, and Srinivasan K (2004) Activities of glycosidases during fruit development and ripening of tomato (Lycopersicum esculantum L.): implication in fruit ripening. Plant Sci 166, 1451–1459.
Jenkinson HF and Shepherd MG (1987) A mutant of Candida albicans deficient in β-N-acetylhexosaminidase (chitobiase). J Gen Microbiol 33, 2097–2106.
Jin YR, Jo YY, Kim KY, Shim JH, Kim YW, and Park RD (2002) Purification and characterization of β-N-acetylhexosaminidase from rice seeds. J Biochem Mol Biol 35, 313–319.
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685.
Li SC and Li YT (1970) Studies on glycosidases of jack bean meal. J Biol Chem 245, 5153–5160.
Mamarabadi M, Jensen DF, and Lübeck M (2009) An N-acetyl-β-d-glucosaminidase gene, cr-nag1, from the biocontrol agent Clonostachys rosea is up-regulated in antagonistic interactions with Fusarium culmorum. Mycol Res 131, 33–43.
Nieder V, Kutzer, M, Kren V, Gallego RG, Kamerling JP, and Elling L (2004) Screening and characterization of β-N-acetylhexosaminidases for the synthesis of nucleotide-activated disaccharides. Enzyme Microbiol Technol 34, 407–414.
Oikawa A, Itoh E, Ishihara A, and Iwamura H (2003) Purification and characterization of β-N-acetylhexosaminidase from maize seedlings. J Plant Physiol 160, 991–999.
Park RD, Jin YR, and Ryu SR (2001a) N-Acetyl-β-d-hexosaminidases in plants. J Chitin Chitosan 6, 143–149.
Park RD, Jo KJ, and Jin YR (2001b) Distribution of N-acetyl-β-d-hexosaminidase in plants. J Chitin Chitosan 6, 30–33.
Pocsi I, Kiss L, and Nanasi P (1990) Studies on the N-acetyl-β-d-hexosaminidase from germinating Lupinus luteus L. seeds I. Purification and characterization. Protein Struct Mol Enzymol 1039, 110–118.
Pusztahelyi T, Posci I, and Szentirmai A (1997) Aging of Penicillium chrysogenum cultures under carbon starvation. II. protease and N-acetyl-β-d-hexosaminidase production. Biotechnol Appl Biochem 25, 87–93.
Ren YY and West CA (1992) Elicitation of diterpene biosynthesis in rice (Oryza sativa L.) by chitin. Plant Physiol 99, 1169–1178.
Truchet G, Roche P, Lerouge P, Vasse J, Camut S, de Billy F et al. (1991) Sulphated lipo-oligosaccharide signals of Rhizobium meliloti elicit root nodule organogenesis in alfalfa. Nature 351, 670–673.
Yamada A, Shibuya N, Kodama O, and Akatsuka T (1993) Induction of phytoalexin formation in suspension-cultured rice cells by N-acetylchitooligosaccharides. Biosci Biotechnol Biochem 57, 405–409.
Yi CK (1981) Increase in β-N-acetylglucosaminidase activity during germination of cotton seeds. Plant Physiol 67, 68–73.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ju, WT., Nguyen, V.N., Jung, WJ. et al. Purification and characterization of a β-N-acetylhexosaminidase from wheat bran and its applicability to biocontrol of Fusarium solani . J Korean Soc Appl Biol Chem 55, 729–735 (2012). https://doi.org/10.1007/s13765-012-2161-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13765-012-2161-y