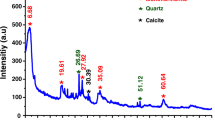
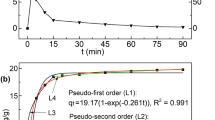
Abstract
Methyl tert-butyl ether (MTBE), an underground water contaminant, has the potential to cause serious health and environmental issues, necessitating its removal before any use of the water. This research investigated the adsorption behavior of MTBE on natural nanoporous adsorbents, namely bentonite clay and clinoptilolite, using an experimental batch system. The optimum conditions for removing MTBE from water were determined by investigating the impact of adsorption parameters, such as the solution pH, temperature, time, MTBE concentration, and the dosage of bentonite clay and clinoptilolite. The removal efficiency of bentonite and clinoptilolite was found to be 98.09% and 97.44%, respectively, with adsorption capacity values of 2220 and 2002.159 mgg−1, respectively. The experimental results were accurately modeled by utilizing the pseudo-second-order equation and the Langmuir model for adsorption kinetics and isotherms, respectively. The thermodynamic analysis, including determination of the change in free energy, enthalpy and entropy under standard condition, indicated an exothermic and spontaneous adsorption process for MTBE utilizing the natural nanoporous adsorbents. At the end, the results demonstrated that both bentonite and clinoptilolite are effective natural adsorbents for removing MTBE from aquatic environments.









Similar content being viewed by others
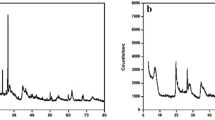
References
Alshahrani F, Tawabini B, Saleh T, Alrayaan M, Alaama S, Nasser R, Soupios P, Kirmizakis P, Mahmoud M, Oyehan T (2022) Removal of benzene, MTBE and toluene from contaminated waters using biochar-based liquid activated carbon. Sci Rep 12:1–11
Angar Y, Djelali N-E, Kebbouche-Gana S (2017) Investigation of ammonium adsorption on Algerian natural bentonite. Environ Sci Pollut Res 24:11078–11089
Behzadnezhad A, Ebadi T, Taheri S, Kowsari E (2020) Batch adsorption of methyl tert-butyl ether (MTBE) from aqueous solution by combined CNT and zeolite. Desalin Water Treat 191:213–220
Borah D, Nath H, Saikia H (2022) Modification of bentonite clay & its applications: a review. Rev Inorg Chem 42:265–282
Camacho LM, Deng S, Parra RR (2010) Uranium removal from groundwater by natural clinoptilolite zeolite: effects of pH and initial feed concentration. J Hazard Mater 175:393–398
Chen D, Zhang J, Chen J (2010) Adsorption of methyl tert-butyl ether using granular activated carbon: equilibrium and kinetic analysis. Int J Environ Sci Technol 7:235–242
Cheng Z, Liu X, Han M, Ma W (2010) Adsorption kinetic character of copper ions onto a modified chitosan transparent thin membrane from aqueous solution. J Hazard Mater 182:408–415
Ekinci EK (2022) Mesoporous magnesia sorbent for removal of organic contaminant methyl tert-butyl ether (MTBE) from water. Sep Sci Technol 57:843–853
Gambhir RS, Kapoor V, Nirola A, Sohi R, Bansal V (2012) Water pollution: impact of pollutants and new promising techniques in purification process. J Hum Ecol 37:103–109
Ghayurdoost F, Assadi A, Mehrasbi MR (2022) Effects of operational parameters on methyl tert-butyl ether removal by permeable reactive barrier from polluted waters. Environ Health Eng Manag 9:271–279
Hua T, Li S, Wang L, Yan W (2023) Enhanced methyl tert-butyl ether removal by mixed consortium: performance and adaptability. Appl Sci 13:2144
Hung H-W, Lin T-F, Baus C, Sacher F, Brauch H (2005) Competitive and hindering effects of natural organic matter on the adsorption of MTBE onto activated carbons and zeolites. Environ Technol 26:1371–1382
Inal F, Yetgin S, Aksu GT, Simsek S, Sofuoglu A, Sofuoglu SC (2009) Activated carbon adsorption of fuel oxygenates MTBE and ETBE from water. Water, Air, Soil Pollut 204:155–163
Inyinbor Adejumoke A, Adebesin Babatunde O, Oluyori Abimbola P, Adelani Akande Tabitha A, Dada Adewumi O, Oreofe Toyin A (2018) Water pollution: effects, prevention, and climatic impact. Water Chall Urban World 33:33–47
Khoshand A, Bazargan A, Rahimi K (2018) Application of soils for removal of methyl tertiary butyl ether (MTBE) from aqueous solution: adsorption kinetics and equilibrium study. Desalin Water Treat 111:226–235
Levchuk I, Bhatnagar A, Sillanpää M (2014) Overview of technologies for removal of methyl tert-butyl ether (MTBE) from water. Sci Total Environ 476:415–433
Li J-j, Zhou F, Tang X-d, Hu T, Cheng J (2016) Experimental study on deep desulfurization of MTBE by electrochemical oxidation and distillation. RSC Adv 6:4803–4809
Lu J, Xu F, Cai W (2008) Adsorption of MTBE on nano zeolite composites of selective supports. Microporous Mesoporous Mater 108:50–55
Mahmoodsaleh F, Roayaei Ardakani M (2022) Methyl tertiary butyl ether biodegradation by the bacterial consortium isolated from petrochemical wastewater and contaminated soils of Imam Khomeini Port petrochemical company (Iran). Bioremed J 26:127–137
Mansouri N, Rikhtegar N, Panahi HA, Atabi F, Shahraki BK (2013) Porosity, characterization and structural properties of natural zeolite-clinoptilolite-as a sorbent. Environ Prot Eng 39:139–152
Mehrjouei M, Müller S, Möller D (2014) Decomposition kinetics of MTBE, ETBE and TAEE in water and wastewater using catalytic and photocatalytic ozonation. J Mol Catal A Chem 386:61–68
Murray HH (2006) Bentonite applications. Develop Clay Sci 2:111–130
Naser Sheykhaoleslami NS, Irani M, Gholamian R, Aliabadi M (2016) Removal of MTBE from aqueous solution using natural nanoclays of Iran. Desalin Water Treat 57:27259–27268
Nikazar M, Gorji LM, Shojae S, Keynejad K, Haghighaty A, Jalili F, Mirzahosseini A (2014) Removal of methyl tertiary-butyl ether (MTBE) from aqueous solution using sunlight and nano TiO2. Energ Source Part A 36:2305–2311
Olegario EM, Gili MBZ, Celikin M (2021) Characterization of Philippine natural bentonite. Exp Results 2:e25
Padmavathy K, Madhu G, Haseena P (2016) A study on effects of pH, adsorbent dosage, time, initial concentration and adsorption isotherm study for the removal of hexavalent chromium (Cr (VI)) from wastewater by magnetite nanoparticles. Proc Technol 24:585–594
Qu C, Liang D-w (2022) Novel electrochemical advanced oxidation processes with H2O2 generation cathode for water treatment: a review. J Environ Chem Eng 10:107896
Rad LR, Anbia M (2021) Zeolite-based composites for the adsorption of toxic matters from water: a review. J Environ Chem Eng 9:106088
Rakshit S, Saha R, Singha A, Seddigi ZSA, Pal SK (2013) Molecular interaction, co-solubilization of organic pollutants and ecotoxicity of a potential carcinogenic fuel additive MTBE in water. J Mol Liq 180:235–243
Safari M, Nikazar M, Dadvar M (2013) Photocatalytic degradation of methyl tert-butyl ether (MTBE) by Fe-TiO2 nanoparticles. J Ind Eng Chem 19:1697–1702
Sahoo TR, Prelot B (2020) Chapter 7 - Adsorption processes for the removal of contaminants from wastewater: the perspective role of nanomaterials and nanotechnology. In: Bonelli B, Freyria FS, Rossetti I, Sethi R (eds) Nanomaterials for the Detection and Removal of Wastewater Pollutants. Elsevier, Amsterdam, pp 161–222
Samaei MR, Maleknia H, Azhdarpoor A (2016) A comparative study of removal of methyl tertiary-butyl ether (MTBE) from aquatic environments through advanced oxidation methods of H2O2/nZVI, H2O2/nZVI/ultrasound, and H2O2/nZVI/UV. Desalin Water Treat 57:21417–21427
Seddigi ZS, Bumajdad A, Ansari SP, Ahmed SA, Danish EY, Yarkandi NH, Ahmed S (2014) Preparation and characterization of Pd doped ceria–ZnO nanocomposite catalyst for methyl tert-butyl ether (MTBE) photodegradation. J Hazard Mater 264:71–78
Singh J, Yadav P, Pal AK, Mishra V (2020) Water pollutants: Origin and status in Sensors in water pollutants monitoring Role of material, Springer, Berlin pp 5–20
Sirait M, Bukit N, Siregar N (2017) Preparation and characterization of natural bentonite in to nanoparticles by co-precipitation method, translated by Makassar, Indonesia: AIP Publishing LLC, 020006
Stefanakis AI (2019) The fate of MTBE and BTEX in constructed wetlands. Appl Sci 10:127
Suzuki M, Yamazaki K, Kano H, Aiso S, Nagano K, Fukushima S (2012) No carcinogenicity of ethyl tertiary-butyl ether by 2-year oral administration in rats. J Toxicol Sci 37:1239–1246
Tanaka H, Yamasaki N, Muratani M, Hino R (2003) Structure and formation process of (K, Na)-clinoptilolite. Mater Res Bull 38:713–722
Vakili M, Rafatullah M, Salamatinia B, Ibrahim MH, Ismail N, Abdullah AZ (2017) Adsorption studies of methyl tert-butyl ether from environment. Sep Purif Rev 46:273–290
Velichenko AB, Dmitrikova LV, Kopteva SD, Korshin GV, Chuvasova NO (2014) Electrochemical degradation of methyl tert-butyl ether. J Chem Technol 22:1–7
Wu T-N (2011) Electrochemical removal of MTBE from water using the iridium dioxide coated electrode. Sep Purif Technol 79:216–220
Zendelska A, Golomeova M, Jakupi S, Lisichkov K, Kuvendziev S, Marinkovski M (2018) Characterization and application of clinoptilolite for removal of heavy metal ions from water resources. Geol Maced 32:21–32
Zhang Y, Jin F, Shen Z, Lynch R, Al-Tabbaa A (2018) Kinetic and equilibrium modelling of MTBE (methyl tert-butyl ether) adsorption on ZSM-5 zeolite: batch and column studies. J Hazard Mater 347:461–469
Zhang Y, Xue K, Li H, Lian S, Han C, Zhu Z, Lu Y, Qi J, Wang Y (2023) Mechanism analysis and liquid-liquid equilibrium of methyl tert-butyl ether separation from petroleum wastewater azeotrope by green mixed solvent. J Environ Chem Eng 11:109389
Zhirong L, Uddin MA, Zhanxue S (2011) FT-IR and XRD analysis of natural Na-bentonite and Cu (II)-loaded Na-bentonite. Spectrochim Acta A Mol Biomol Spectrosc 79:1013–1016
Acknowledgements
The authors would like to extend their appreciation to the laboratory staff at Chemical Engineering Department, University of Mohaghegh Ardabili, for their assistance in facilitating the experimental work.
Funding
There was no external funding for this study.
Author information
Authors and Affiliations
Contributions
NS contributed to experimental work, writing—original draft. BM contributed to conceptualization, writing—editing, and supervision. FHS contributed to English editing, validation, data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of/competing interests.
Ethical approval
Ethical approval is not applicable for this article.
Consent to participate
Consent to participate is not applicable for this article.
Consent to publish
Consent to publish is not applicable for this article.
Additional information
Editorial responsibility: Yuhang Yang.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shojaeifar, N., Mirzayi, B. & Saboor, F.H. Highly efficient removal of MTBE using natural nanoporous adsorbents. Int. J. Environ. Sci. Technol. 21, 6553–6566 (2024). https://doi.org/10.1007/s13762-024-05497-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-024-05497-9