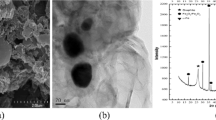
Abstract
Citrus peels, as inevitable waste product of the citrus processing industry, cause major pollution problems in the environment. These waste products have the potential to produce zero-valent iron nanoparticles, which have been successful in treating different groups of pollutants. Sediment contamination with metals is very challenging in finding effective solution for reducing the contribution of toxicity to the environment. Thus, it is crucial to involve remediation technique as stabilization which includes the addition of inorganic amendments in order to immobilize metals from the sediment. The study demonstrates the issue with Begej sediment that has been contaminated with toxic metals and the possibility of its immobilization using biosynthesized zero-valent iron nanoparticles from citrus peel extracts. Leaching tests are used to demonstrate the benefits and drawbacks of applied treatment for forecasting the behavior of contaminants. The toxicity test, using Vibrio fisheri bacteria, is used to evaluate whether the sediment samples show inhibitory effects. The obtained treated mixtures of sediment and biosynthesized zero-valent iron nanoparticles are determined to be non-hazardous wastes because the concentrations of leached metals (Cu, Cr, Ni and Cd) have fallen below the established maximum values prescribed by German Standard Procedure for Water, Wastewater and Sediment Testing and Toxicity Characteristic Leaching Procedure. Additionally, toxicity test using Vibrio fisheri bacteria has shown that treated sediment is nontoxic. These findings represent promising results in application of zero-valent iron nanoparticles biosynthesized using citrus peel extracts for removal of inorganic pollutants and also create treated sediment for potential further beneficial sustainable use.





Similar content being viewed by others
Abbreviations
- AAS:
-
Atomic absorption spectroscopy
- CDI:
-
Chronic daily intake
- CR:
-
Carcinogenic risk
- DO:
-
Dry orange peel
- DO-nZVIs:
-
Dry orange peel zero-valent iron nanoparticles
- ERL:
-
Effects range-low
- ERM:
-
Effect range-median
- FO:
-
Fresh orange peel
- FO-nZVIs:
-
Fresh orange peel zero-valent iron nanoparticles
- FRAP:
-
Ferric reducing antioxidant power
- FTIR:
-
Fourier-transform infrared spectroscopy
- GCF:
-
Global contamination factor
- HI:
-
Hazard index
- HQ:
-
Hazard quotient
- HQderm :
-
Hazard quotient for dermal contact
- HQing :
-
Hazard quotient for exposure by ingestion
- HQinh :
-
Hazard quotient for exposure by inhalation
- ICF:
-
Individual contamination factor
- nZVIs:
-
Zero-valent iron nanoparticles
- O:
-
Orange
- PEL:
-
Probable effect level
- PO:
-
Pomelo peel
- PO-nZVIs:
-
Pomelo peel zero-valent iron nanoparticles
- RfD:
-
Reference doses
- SEM/EDS:
-
Scanning electron microscopy/energy-dispersive spectroscopy
- SKGs:
-
Sediment quality guidelines
- TEL:
-
Threshold effect level
References
Abdallah MA (2017) Chemical speciation and contamination assessment of Pb and V by sequential extraction in surface sediment off Nile delta. Egypt Arab J Chem 10:68–75. https://doi.org/10.1016/j.arabjc.2012.06.001
Ali KA, Yao R, Wu W, Masum MMI, Luo J, Wang Y, Zhang Y, An Q, Sun G, Li B (2020) Biosynthesis of silver nanoparticle from pomelo (Citrus Maxima) and their antibacterial activity against Acidovorax oryzae RS-2. Mater Res Express 7:015097. https://doi.org/10.1088/2053-1591/ab6c5e
Aranda A, Berenice C (2008) Leaching test comparison for solidified and stabilized contaminated sediments: assessment of selected inorganic contaminants. Master Thesis in Geosciences, Faculty of Mathematics and Natural Sciences, University of Oslo, Norway
ASTM D1557-00 Standard test method for laboratory compaction characteristics of soil using modified effort American Society for Testing Materials. Annual book of ASTM standards: ASTM D1557-91, vol 4.08. ASTM, Philadelphia
Bashir M, Ali S, Farrukh MA (2020) Green synthesis of Fe2O3 nanoparticles from orange peel extract and a study of its antibacterial activity. J Korean Phys Soc 76:848–854. https://doi.org/10.3938/jkps.76.848
Borja JQ, Ngo MAS, Saranglao CC, Tiongco RPM, Roque EC, Dugos NP (2015) Synthesis of green zero-valent iron using polyphenols from dried green tea extract. JESTEC, School of Engineering, Taylor’s University, p 22–31
DIN 38414–4 (1984) Teil 4: Schlamm und Sedimente, Gruppe S., Bestimmung der Eluierbarkeit mit Wasser S4, Beuth Verlag, Berlin
Du C, Chen H, Gao W, Sun W, Peng L, Xu N (2023) Green synthesis of nano-zero valence iron with green tea and it’s implication in lead removal. Bull Environ Contam Toxicol 110:10. https://doi.org/10.1007/s00128-022-03649-6
Dubovina M, Krčmar D, Grba N, Watson MA, Rađenović D, Tomašević-Pilipović D, Dalmacija B (2018) Distribution and ecological risk assessment of organic and inorganic pollutants in the sediments of the transnational Begej canal (Serbia-Romania). Environ Pollut 236:773–784. https://doi.org/10.1016/j.envpol.2018.02.014
Fazzino F, Pedullà A, Calabrò P (2023) Boosting the circularity of waste management: pretreated mature landfill leachate enhances the anaerobic digestion of market waste. Biofuel Res J 10(1):1764–1773. https://doi.org/10.18331/BRJ2023.10.1.2
Francy N, Shanthakumar S, Chiampo F, Sekhar YR (2020) Remediation of lead and nickel contaminated soil using nanoscale zero-valent iron (nZVI) particles synthesized using green leaves: first results. Processes 8:1453. https://doi.org/10.3390/pr8111453
Gao H, Tao H, Yang Y, Che Q, Tang Q, Gu Y (2023) Effect of humus on the solidification and stabilization of heavy metal contaminated river sediment. Int J Environ Res Public Health 20:4882. https://doi.org/10.3390/ijerph20064882
Gupta VK, Nayak A (2012) Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. J Chem Eng 180:81–90. https://doi.org/10.1016/j.cej.2011.11.006
Huang D, Hu Z, Peng Z, Zeng G, Chen G, Zhang C, Cheng M, Wan J, Wang X, Qin X (2018) Cadmium immobilization in river sediment using stabilized nanoscale zero-valent iron with enhanced transport by polysaccharide coating. J Environ Econ Manag 210:191–200. https://doi.org/10.1016/j.jenvman.2018.01.001
Huang L, Weng X, Chen Z, Megharaj M, Naidu R (2014) Green synthesis of iron nanoparticles by various tea extracts: comparative study of the reactivity. Spectrochim Acta A Mol Biomol Spectrosc 130:295–301. https://doi.org/10.1016/j.saa.2014.04.037
ISO 11277 (2009) Soil quality—determination of particle size distribution in mineral soil material—method by sieving and sedimentation
ISO 11348–1 (2008) Water quality—determination of the inhibitory effect of water samples on the light emission of Vibrio fischeri (Luminiscent Bacteria Test); British Standards Institution: London, UK, 2008
ISO (2005) International organization for standardization, ISO 14502–1. Determination of substances characteristics of green and black tea- part 1: content of total polyphenols in tea- colorimetric method using Folin- Ciocalteu reagent. p 14
Jagwani D, Krishna PH (2021) Nature’s nano-assets: green synthesis, characterization techniques and applications—a graphical review. Mater Today 46:2307–2317. https://doi.org/10.1016/j.matpr.2021.04.185
Jain CK (2004) Metal fractionation study on bed sediments of river Yamuna. India Water Res 38:569–578. https://doi.org/10.1016/j.watres.2003.10.042
Jarque S, Masner P, Klánová J, Prokeš R, Bláha L (2016) Bioluminescent Vibrio fischeri assays in the assessment of seasonal and spatial patterns in toxicity of contaminated river sediments. Front Microbiol 7:1738. https://doi.org/10.3389/fmicb.2016.01738
Jia S, Tian Y, Zheng B, Song Y, He N, Peng Z, Peng S, Zhao W (2023) Pollution characteristics of full-scale butterfly valves: Risk assessment of heavy metal release behavior influenced by the bacterial communities and corrosion products. Bioresour Technol 21:101331. https://doi.org/10.1016/j.biteb.2023.101331
Kumar B, Smita K, Galeas S, Sharma V, Guerrero VH, Debut A, Cumbal L (2020) Characterization and application of biosynthesized iron oxide nanoparticles using Citrus paradisi peel: a sustainable approach. Inorg Chem Commun 119:108116. https://doi.org/10.1016/j.inoche.2020.108116
Lin J, He F, Su B, Sun M, Owens G, Chen Z (2019) The stabilizing mechanism of cadmium in contaminated soil using green synthesized iron oxide nanoparticles under long-term incubation. J Hazard Mater 379:120832. https://doi.org/10.1016/j.jhazmat.2019.120832
Lingamdinne LP, Vemula KR, Chang YY, Yang JK, Karri RR, Koduru JR (2020) Process optimization and modeling of lead removal using iron oxide nanocomposites generated from bio-waste mass. Chemosphere 243:125257. https://doi.org/10.1016/j.chemosphere.2019.125257
Liu L, Li W, Song W, Guo M (2018) Remediation techniques for heavy metal-contaminated soils: principles and applicability. Sci Total Environ 633:206–219. https://doi.org/10.1016/j.scitotenv.2018.03.161
López-Téllez G, Balderas-Hernández P, Barrera-Díaz CE, Vilchis-Nestor AR, Roa-Morales G, Bilyeu B (2013) Green method to form iron oxide nanorods in orange peels for chromium(VI) reduction. J Nanosci Nanotechnol 13(3):2354–2361. https://doi.org/10.1166/jnn.2013.7093
Machado S, Grosso JP, Nouws HP, Albergaria JT, Delerue MC (2014) Utilization of food industry wastes for the production of zero-valent iron nanoparticles. Sci Total Environ 496:233–240. https://doi.org/10.1016/j.scitotenv.2014.07.058
Machado S, Pacheco JG, Nouws HPA, Albergaria JT, Delerue-Matos C (2015) Characterization of green zero-valent iron nanoparticles produced with tree leaf extracts. Sci Total Environ 533:76–81. https://doi.org/10.1016/j.scitotenv.2015.06.091
Mystrioti C, Sparis D, Papasiopi N, Xenidis A, Dermatas D, Chrysochoou M (2015) Assessment of polyphenol coated nano zero valent iron for hexavalent chromium removal from contaminated waters. Bull Environ Contam Toxicol 94:302–307. https://doi.org/10.1007/s00128-014-1442-z
Naji A, Ismail A, Ismail AR (2010) Chemical speciation and contamination assessment of Zn and Cd by sequential extraction in surface sediment of Klang river. Malays Microchem J 95:285–292. https://doi.org/10.1016/j.microc.2009.12.015
Official Gazette Ministry of Energy (2010) Development and the Environment, Regulation on categories, testing and classification of waste. The Official Gazette No. 56/2010
Official Gazette Ministry of Housing (2002) Spatial planning and environment directorate-general for environmental protection: circular on target values and intervention values for soil remediation, Netherlands Government. Gazette No. 39/2000
Official Gazette (2012)Regulation on limit values for pollutants in surface and groundwaters and sediments, and the deadlines for their achievement. Belgrade, Serbia. Gazette No. 50/2012
Parvez S, Venkataraman C, Mukherji S (2006) A review on advantages of implementing luminescence inhibition test (Vibrio fischeri) for acute toxicity prediction of chemicals. Environ Int 32:265–268. https://doi.org/10.1016/j.envint.2005.08.022
Pulido R, Bravo L, Sauro-Calixo F (2000) Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem 48:3396–3402. https://doi.org/10.1021/jf9913458
Salem SS, Badawy MSEM, Al-Askar AA, Arishi AA, Elkady FM, Hashem AH (2022) Green biosynthesis of selenium nanoparticles using orange peel waste: characterization, antibacterial and antibiofilm activities against multidrug-resistant bacteria. Life 12(6):893. https://doi.org/10.3390/life12060893
Salmani MH, Abedi M, Mozaffari SA, Mahvi AH, Sheibani A, Jalili M (2021) Simultaneous reduction and adsorption of arsenite anions by green synthesis of iron nanoparticles using pomegranate peel extract. J Environ Health Sci Eng 19:603–612. https://doi.org/10.1007/s40201-021-00631-y
Skiba MI, Vorobyova VI (2019) Synthesis of silver nanoparticles using orange peel extract prepared by plasmochemical extraction method and degradation of methylene blue under solar irradiation. Adv Mater Sci Eng 115:1–8. https://doi.org/10.1155/2019/8306015
Slijepčević N, Kerkez Đ, Tomašević Pilipović D, Bečelić-Tomin M, Krčmar D (2018) Use of two different approaches to the synthesis of nano zero valent iron for sediment remediation. Glob NEST J 21(4):455–460. https://doi.org/10.30955/gnj.002649
Slijepčević N, Tomašević Pilipović D, Kerkez Đ, Krčmar D, Bečelić-Tomin M, Beljin J, Dalmacija B (2021) A cost effective method for immobilization of Cu and Ni polluted river sediment with nZVI synthesized from leaf extract. Chemosphere 263:127816. https://doi.org/10.1016/j.chemosphere.2020.127816
Spence RD, Shi C (2005) Stabilization and solidification of hazardous, radioactive and mixed wastes. CRC Press, Boca-Raton
SRPS EN 12879 (2007)-Determination of organic matter content in soil as loss-on-ignition
SRPS ISO 10390 (2007)-Determination of pH value in soil
SRPS ISO 5667-12-Water quality—sampling-part 12: guidance on sampling of sediment from riverbeds, lakes and estuaries
Su B, Lin J, Owens G, Chen Z (2020) Impact of green synthesized iron oxide nanoparticles on the distribution and transformation of As species in contaminated soil. Environ Pollut 258:113668. https://doi.org/10.1016/j.envpol.2019.113668
Ting AS, Chin JE (2020) Biogenic synthesis of iron nanoparticles from apple peel extracts for decolorization of malachite green dye. Water Air Soil Poll 231:278. https://doi.org/10.1007/s11270-020-04658-z
Tran NDN, Bui TH, Nguyen AP, Nguyen T-T, Nguyen VM, Duong NL, Nguyen T (2022) The ability of silver biochar green-synthesized from Citrus maxima peel to adsorb pollutant organic compounds and antibacterial activity. Green Chem Lett Rev 15(1):18–27. https://doi.org/10.1080/17518253.2021.2015456
Trung ND, Phuong Tung LN, Hong NT (2022) Synthesis of silver nanoparticles using extract of Citrus maxima peel. CTUJS 14(2):93–98. https://doi.org/10.22144/ctu.jen.2022.014
USEPA (2007) Method 3051a, Microwave assisted acid digestion of sediments, sludges, soils and, Revision 1
USEPA (2007) Method 7000B. Flame atomic absorption spectrophotometry, Revision 2
USEPA (2007) Method 7010. Graphite furnace absorption spectrophotometry, Revision 0
USEPA (1986) Superfund Public Health Evaluation Manual; U.S. Environmental Protection Agency: Washington, DC, USA, p 1–86
USEPA (1989). Human Health Evaluation Manual. In Risk Assessment Guidance for Superfund; EPA/540/1-89/002; Office of Emergency and Remedial Response, U.S. Environmental Protection Agency: Washington, DC, USA, Volume 1
USEPA (2001). Baseline Human Health Risk Assessment Vasquez Boulevard and I-70 Superfund Site Denver, Co; U.S. Environmental Protection Agency: Washington, DC, USA
USEPA (2002). Toxicity characteristic leaching procedure, method 1311. www.EPA.gov/SW-846/1311.pdf
USEPA (1997) Exposure Factors Handbook EPA/600/P-95/002F; National Center for Environmental Assessment, US EPA Office of Research and Development: Washington, DC, USA
USEPA (2011) Exposure Factors Handbook. Edition, US Environmental Protection Agency, Washington, DC, USA
Wang L, Chen L, Cho DW, Tsang DCW, Yang J, Hou D, Baek K, Kua HW, Poon CS (2019) Novel synergy of Si-rich minerals and reactive MgO for stabilisation/solidification of contaminated sediment. J Hazard Mater 365:695–706. https://doi.org/10.1016/j.jhazmat.2018.11.067
Wei Y, Fang Z, Zheng L, Tan L, Tsang EP (2016) Green synthesis of Fe nanoparticles using Citrus maxima peels aqueous extracts. Mater Lett 185:384–386. https://doi.org/10.1016/j.matlet.2016.09.029
Wen J, Yi YJ, Zeng GM (2016) Effects of modified zeolite on the removal and stabilization of heavy metals in contaminated lake sediment using BCR sequential extraction. J Environ Manag 178:63–69. https://doi.org/10.1016/j.jenvman.2016.04.046
Weng X, Huang L, Chen Z, Megharaj M, Naidu R (2013) Synthesis of iron-based nanoparticles by green tea extract and their degradation of malachite. Ind Crops Prod 51:342–347. https://doi.org/10.1016/j.indcrop.2013.09.024
Xiao L, Ye F, Zhou Y, Zhao G (2021) Utilization of pomelo peels to manufacture value-added products: a review. Food Chem 351:129247. https://doi.org/10.1016/j.foodchem.2021.129247
Xu W, Yang T, Liu S, Du L, Chen Q, Li X, Dong J, Zhang Z, Lu S, Gong Y, Zhou L, Liu Y, Tan X (2022) Insights into the synthesis, types and application of iron nanoparticles: the overlooked significance of environmental effects. Environ Int 158:106980. https://doi.org/10.1016/j.envint.2021.106980
Xue W, Peng Z, Huang D, Zeng G, Wan J, Xu R, Cheng M, Zhang C, Jiang D, Hu Z (2018) Nanoremediation of cadmium contaminated river sediments: microbial re-sponse and organic carbon changes. J Hazard Mater 359:290–299. https://doi.org/10.1016/j.jhazmat.2018.07.062
Yin Z, Song L, Song H, Hui K, Lin Z, Wang Q, Xuan L, Wang Z, Gao W (2020) Remediation of copper contaminated sediments by granular activated carbon-supported titanium dioxide nanoparticles: mechanism study and effect on enzyme activities. Sci Total Environ 741:139962. https://doi.org/10.1016/j.scitotenv.2020.139962
Yuan CG, Huo C, Gui B, Cao WP (2017) Green synthesis of gold nanoparticles using Citrus maxima peel extract and their catalytic/antibacterial activities. IET Nanobiotechnol 11(5):523–530. https://doi.org/10.1049/iet-nbt.2016.0183
Zapata B, Balmaseda J, Fregoso-Israel E, Torres-Garcia E (2009) Thermo-kinetics study of orange peel in air. J Therm Anal Calorim 98(1):309–315. https://doi.org/10.1007/s10973-009-0146-9
Zhang W, Li SQ, Fu JB (2021) An improved method of solidifying heavy metals in river-lake sediment. J Yangtze River Sci Res Inst 38:15–120. https://doi.org/10.11988/ckyyb.201915802021
Acknowledgements
The research has been financed by the Science Fund of the Republic of Serbia, #7753609, BEuSED.
Author information
Authors and Affiliations
Contributions
NS helped in investigation, writing—original Draft, conceptualization; DR helped in methodology, visualization, writing—review & editing; AKM contributed to methodology, formal analysis; ES performed data curation, formal analysis; ĐK contributed to data curation, formal analysis, writing—review & editing; ALM helped in methodology; writing—review & editing; DTP done writing—review & editing, resources, project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there were known forms of competing or financial interest that could have appeared to influence this study.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Editorial responsibility: Maryam Shabani.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Slijepčević, N., Tomašević Pilipović, D., Rađenović, D. et al. The application of iron nanoparticles biosynthesized using citrus peel extracts for immobilization of metal-contaminated river sediment. Int. J. Environ. Sci. Technol. 21, 3999–4012 (2024). https://doi.org/10.1007/s13762-023-05241-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-05241-9