Abstract
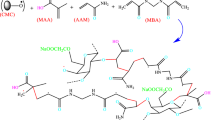
In this study, we synthesized carboxymethyl cellulose-graft-poly(methacrylic acid-co-acrylamide) copolymer hydrogels and carboxymethyl cellulose-graft-poly(methacrylic acid-co-acrylamide)/kaolin nanocomposite with a free radical mechanism to adsorb pollutants of Nile blue dye. Nanocomposite hydrogels performed best at pH = 11, a temperature of 25 °C, a contact time of 90 min, a concentration of pollutant 10 ppm, and an adsorbent dose of 1.5 mg. The increased adsorption rate of NB dye pollutants after adding kaolin-type clay nanoparticles from 96.49 to 98.91% showed the successful addition of nanoparticles to hydrogel copolymer structures. The best isotherm performance was the Langmuir isotherm model, and maximum adsorption capacity for carboxymethyl cellulose-graft-poly(methacrylic acid-co-acrylamide) copolymer hydrogels and for carboxymethyl cellulose-graft-poly(methacrylic acid-co-acrylamide) nanocomposite hydrogel was 138.49 and 149.82, respectively. In the analysis of kinetic models, Elovich's kinetic model had the best performance, where the α value for carboxymethyl cellulose-graft-poly(methacrylic acid-co-acrylamide) copolymer hydrogels are 0.783 and for carboxymethyl cellulose-graft-poly(methacrylic acid-co-acrylamide)/kaolin nanocomposite hydrogel obtained a value of 9.58, which indicates a high adsorption value. On the other hand, the enthalpy parameters (ΔH°) for adsorption process using carboxymethyl cellulose-graft-poly(methacrylic acid-co-acrylamide) and carboxymethyl cellulose-graft-poly(methacrylic acid-co-acrylamide)/kaolin nanocomposite hydrogels were − 61.512 kJ/mol and − 77.281 kJ/mol, respectively. The negativity of ΔH° indicates that the process is exothermic at 5–50 °C using both adsorbers. Furthermore, this process could be spontaneous because the Gibbs free energy (ΔG°) for adsorption processes was negative in experiment temperature range.






Similar content being viewed by others
Abbreviations
- A T :
-
Constants of Temkin model (l/g)
- α :
-
Initial sorption value (mg/g min)
- b T :
-
Constant of Freundlich model (kJ/mol)
- β :
-
Desorption constant (g/mg)
- C 0 :
-
Initial concentration of the contaminant
- C t :
-
Final concentration
- CPMA:
-
CMC-g-P(MAA-co-AAm)
- CPMAK:
-
CMC-g-P(MAA-co-AAm)/kaolin
- ε :
-
Absolute temperature (K)
- k F :
-
Constant of Freundlich model
- k 1 :
-
Pseudo-first-order
- k 2 :
-
Pseudo-second-order (g/mg min)
- K D :
-
Equilibrium constant (KD = 1000 qe/Ce)
- k L :
-
Langmuir adsorption constant
- m :
-
Amount of adsorbent (g L−1)
- n :
-
Constant of Freundlich model
- q ti :
-
Adsorption capacity at time t (mg/g)
- q ei :
-
Adsorption capacity at equilibrium (min−1)
- q m :
-
Maximum adsorption capacity (mg/g)
- q e :
-
Equilibrium adsorption capacity (mg/g)
- R :
-
Universal constant of gases (8.314 J/mol.K)
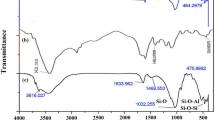
- SEM:
-
Scanning electron microscope
- T :
-
Absolute temperature (K)
- V :
-
Volume of solution
- W dry :
-
Dry weights of hydrogels
- W wet :
-
Wet weights of hydrogels
References
Abou Taleb MF, Abou El Fadl FI, Albalwi H (2021) Adsorption of toxic dye in wastewater onto magnetic NVP/CS nanocomposite hydrogels synthesized using gamma radiation. Sep Purif Technol 266:118551
Ahmadi A, Foroutan R, Esmaeili H, Tamjidi S (2020) The role of bentonite clay and bentonite clay@ MnFe2O4 composite and their physico-chemical properties on the removal of Cr (III) and Cr (VI) from aqueous media. Environ Sci Pollut Res 27:14044–14057
Ai L, Huang H, Chen Z, Wei X, Jiang J (2010) Activated carbon/CoFe2O4 composites: facile synthesis, magnetic performance and their potential application for the removal of malachite green from water. Chem Eng J 156:243–249
Akter M, Bhattacharjee M, Dhar AK, Rahman FBA, Haque S, Rashid TU, Kabir SF (2021) Cellulose-based hydrogels for wastewater treatment: a concise review. Gels 7:30
Al-Aidy H, Amdeha E (2021) Green adsorbents based on polyacrylic acid-acrylamide grafted starch hydrogels: the new approach for enhanced adsorption of malachite green dye from aqueous solution. Int J Environ Anal Chem 101:2796–2816
Alali AF, Almojil SF, Almohana AI, Anqi AE, Rajhi AA, Alamri S, Dhahad HA (2023) Hydroxyapatite@ Mn–Fe composite as a reusable sorbent for removal of Nile blue dye and Cr (VI) from polluted water. Environ Sci Pollut Res 30:18419–18437
Bai T, Zhao K, Gao Q, Qi M, Zhang Y, Lu Z, Zhao H, Gao H, Wei J (2020) Kaolin/CaAlg hydrogel thin membrane with controlled thickness, high mechanical strength, and good repetitive adsorption performance for dyes. Ind Eng Chem Res 59:4958–4967
Batool M, Khurshid S, Qureshi Z, Daoush WM (2021) Adsorption, antimicrobial and wound healing activities of biosynthesised zinc oxide nanoparticles. Chem Pap 75:893–907
Biedrzycka A, Skwarek E, Hanna UM (2021) Hydroxyapatite with magnetic core: synthesis methods, properties, adsorption and medical applications. Adv Coll Interface Sci 291:102401
Chatkin J, Correa L, Santos U (2021) External environmental pollution as a risk factor for asthma. Clin Rev Allergy Immunol 87:1–18
Cui H, Zhang X, Zhang J, Zhang Y (2019) Nanomaterials-based gas sensors of SF6 decomposed species for evaluating the operation status of high-voltage insulation devices. High Volt 4:242–258
Doberenz F, Zeng K, Willems C, Zhang K, Groth T (2020) Thermoresponsive polymers and their biomedical application in tissue engineering—a review. J Mater Chem B 8:607–628
Eltaweil A, Mohamed HA, Abd El-Monaem EM, El-Subruiti G (2020) Mesoporous magnetic biochar composite for enhanced adsorption of malachite green dye: characterization, adsorption kinetics, thermodynamics and isotherms. Adv Powder Technol 31:1253–1263
Esvandi Z, Foroutan R, Mirjalili M, Sorial GA, Ramavandi B (2019) Physicochemical behavior of Penaeuse semisulcatuse chitin for Pb and Cd removal from aqueous environment. J Polym Environ 27:263–274
Fried R, Oprea I, Fleck K, Rudroff F (2022) Biogenic colourants in the textile industry—a promising and sustainable alternative to synthetic dyes. Green Chem 24:13–35
García FE, Plaza-Cazón J, Montesinos VN, Donati ER, Litter MI (2018) Combined strategy for removal of Reactive Black 5 by biomass sorption on Macrocystis pyrifera and zerovalent iron nanoparticles. J Environ Manag 207:70–79
Han D, Zhao H, Gao L, Qin Z, Ma J, Han Y, Jiao T (2021) Preparation of carboxymethyl chitosan/phytic acid composite hydrogels for rapid dye adsorption in wastewater treatment. Colloids Surf A 628:127355
Hasan MM, Shenashen M, Hasan MN, Znad H, Salman MS, Awual MR (2021) Natural biodegradable polymeric bioadsorbents for efficient cationic dye encapsulation from wastewater. J Mol Liq 323:114587
Hu X, Hu Y, Xu G, Li M, Zhu Y, Jiang L, Tu Y, Zhu X, Xie X, Li A (2020) Green synthesis of a magnetic β-cyclodextrin polymer for rapid removal of organic micro-pollutants and heavy metals from dyeing wastewater. Environ Res 180:108796
Islam MA, Ali I, Karim SA, Firoz MSH, Chowdhury A-N, Morton DW, Angove MJ (2019) Removal of dye from polluted water using novel nano manganese oxide-based materials. J Water Process Eng 32:100911
Kiani G, Dostali M, Rostami A, Khataee AR (2011) Adsorption studies on the removal of Malachite Green from aqueous solutions onto halloysite nanotubes. Appl Clay Sci 54:34–39
Kumar V, Rehani V, Kaith BS (2018) Synthesis of a biodegradable interpenetrating polymer network of Av-cl-poly (AA-ipn-AAm) for malachite green dye removal: kinetics and thermodynamic studies. RSC Adv 8:41920–41937
Li J, Kaku T, Tokura Y, Matsukawa K, Homma K, Nishimoto T, Hiruta Y, Akimoto AM, Nagase K, Kanazawa H (2019a) Adsorption–desorption control of fibronectin in real time at the liquid/polymer interface on a quartz crystal microbalance by thermoresponsivity. Biomacromol 20:1748–1755
Li W, Mu B, Yang Y (2019b) Feasibility of industrial-scale treatment of dye wastewater via bio-adsorption technology. Biores Technol 277:157–170
Li Y, Hou X, Pan Y, Wang L, Xiao H (2020) Redox-responsive carboxymethyl cellulose hydrogel for adsorption and controlled release of dye. Eur Polym J 123:109447
Liu K, Lin B (2019) Research on influencing factors of environmental pollution in China: a spatial econometric analysis. J Clean Prod 206:356–364
Long C, Jiang Z, Shangguan J, Qing T, Zhang P, Feng B (2021) Applications of carbon dots in environmental pollution control: a review. Chem Eng J 406:126848
Lum P, Foo K, Zakaria N, Palaniandy P (2020) Ash based nanocomposites for photocatalytic degradation of textile dye pollutants: a review. Mater Chem Phys 241:122405
Marcus RN, Joseph CG, Taufiq-Yap YH, Soloi S, Abd Majid MH, Jamain Z, Mohd Ali SA, Vijayan V, Pang CK (2022) Current trends on the utilization of ozonation treatment process for the remediation of dye wastewater: a short review
Marouch S, Benbellat N, Duran A, Yilmaz E (2022) Nanoclay-and TiO2 nanoparticle-modified poly(N-vinyl pyrrolidone) hydrogels: a multifunctional material for application in photocatalytic degradation and adsorption-based removal of organic contaminants. ACS Omega 7:35256–35268
Mirzaei M, Habibi MH, Sabzyan H (2021) Synthesis, characterization, and dye degradation photocatalytic activity of the nano-size copper iron binary oxide. Environ Sci Pollut Res 514:1–20
Mittal H, Al Alili A, Alhassan SM (2020) High efficiency removal of methylene blue dye using κ-carrageenan-poly (acrylamide-co-methacrylic acid)/AQSOA-Z05 zeolite hydrogel composites. Cellulose 27:8269–8285
Montoya Salazar JM (2022) Caracterización de nanoandamios sintetizados a partir de polihidroxibutirato (PHB) modificados con cristal bioactivo a tres diferentes proporciones
Nayak AK, Pal A (2020) Statistical modeling and performance evaluation of biosorptive removal of Nile blue A by lignocellulosic agricultural waste under the application of high-strength dye concentrations. J Environ Chem Eng 8:103677
Ninciuleanu CM, Ianchiș R, Alexandrescu E, Mihăescu CI, Burlacu S, Trică B, Nistor CL, Preda S, Scomoroscenco C, Gîfu C (2022) Adjusting some properties of poly(methacrylic acid)(nano) composite hydrogels by means of silicon-containing inorganic fillers. Int J Mol Sci 23:10320
Olad A, Eslamzadeh M, Mirmohseni A (2019) Ion crosslinked poly(acrylic acid-co-acrylamide)/poly (vinyl alcohol)/Cloisite 15 A nanocomposite hydrogels as potential wound dressing films: effect of clay content on water absorption kinetic and mechanical properties. Polym Compos 40:1762–1773
Peighambardoust SJ, Aghamohammadi-Bavil O, Foroutan R, Arsalani N (2020) Removal of malachite green using carboxymethyl cellulose-g-polyacrylamide/montmorillonite nanocomposite hydrogel. Int J Biol Macromol 159:1122–1131
Qiao L, Wang T, Liao Y, Du K (2022) Macroporous chitin microspheres prepared by surfactant micelle swelling strategy for rapid capture of lead (II) from wastewater. Carbohyd Polym 276:118775
Rabipour M, Sekhavat Pour Z, Sahraei R, Ghaemy M, Erfani Jazi M, Mlsna TE (2020) pH-sensitive nanocomposite hydrogels based on poly(vinyl alcohol) macromonomer and graphene oxide for removal of cationic dyes from aqueous solutions. J Polym Environ 28:584–597
Rahmati M, Silva EA, Reseland JE, Heyward CA, Haugen HJ (2020) Biological responses to physicochemical properties of biomaterial surface. Chem Soc Rev 49:5178–5224
Ranjbari S, Tanhaei B, Ayati A, Khadempir S, Sillanpää M (2020) Efficient tetracycline adsorptive removal using tricaprylmethylammonium chloride conjugated chitosan hydrogel beads: Mechanism, kinetic, isotherms and thermodynamic study. Int J Biol Macromol 155:421–429
Safarzadeh H, Peighambardoust SJ, Mousavi SH, Foroutan R, Mohammadi R, Peighambardoust SH (2022a) Adsorption ability evaluation of the poly (methacrylic acid-co-acrylamide)/cloisite 30B nanocomposite hydrogel as a new adsorbent for cationic dye removal. Environ Res 212:113349
Safarzadeh H, Peighambardoust SJ, Mousavi SH, Mohammadi R, Peighambardoust SH (2022b) Adsorption of methyl violet dye from wastewater using poly (methacrylic acid-co-acrylamide)/bentonite nanocomposite hydrogels. J Polym Res 29:113
Safarzadeh H, Peighambardoust SJ, Peighambardoust SH (2023) Application of a novel sodium alginate-graft-poly (methacrylic acid-co-acrylamide)/montmorillonite nanocomposite hydrogel for removal of malachite green from wastewater. J Polym Res 30:157
SARı, A. & TUZEN, M. (2009) Equilibrium, thermodynamic and kinetic studies on aluminum biosorption from aqueous solution by brown algae (Padina pavonica) biomass. J Hazard Mater 171:973–979
Shafiee M, Foroutan R, Fouladi K, Ahmadlouydarab M, Ramavandi B, Sahebi S (2019) Application of oak powder/Fe3O4 magnetic composite in toxic metals removal from aqueous solutions. Adv Powder Technol 30:544–554
Shahbazi M, Jäger H, Ahmadi SJ, Lacroix M (2020) Electron beam crosslinking of alginate/nanoclay ink to improve functional properties of 3D printed hydrogel for removing heavy metal ions. Carbohyd Polym 240:116211
Shahinpour A, Tanhaei B, Ayati A, Beiki H, Sillanpää M (2022) Binary dyes adsorption onto novel designed magnetic clay-biopolymer hydrogel involves characterization and adsorption performance: Kinetic, equilibrium, thermodynamic, and adsorption mechanism. J Mol Liq 366:120303
Sheth Y, Dharaskar S, Chaudhary V, Khalid M, Walvekar R (2022) Prospects of titanium carbide-based MXene in heavy metal ion and radionuclide adsorption for wastewater remediation: a review. Chemosphere 293:133563
Shokry H, Elkady M, Hamad H (2019) Nano activated carbon from industrial mine coal as adsorbents for removal of dye from simulated textile wastewater: operational parameters and mechanism study. J Market Res 8:4477–4488
Sirajudheen P, Karthikeyan P, Ramkumar K, Meenakshi S (2020) Effective removal of organic pollutants by adsorption onto chitosan supported graphene oxide-hydroxyapatite composite: A novel reusable adsorbent. J Mol Liq 318:114200
Velusamy S, Roy A, Sundaram S, KumarMallick T (2021) A review on heavy metal ions and containing dyes removal through graphene oxide-based adsorption strategies for textile wastewater treatment. Chem Rec 21:1570–1610
Wang F, Li J, Su Y, Li Q, Gao B, Yue Q, Zhou W (2019) Adsorption and recycling of Cd (II) from wastewater using straw cellulose hydrogel beads. J Ind Eng Chem 80:361–369
Wang T, Yu C, Chu Q, Wang F, Lan T, Wang J (2020) Adsorption behavior and mechanism of five pesticides on microplastics from agricultural polyethylene films. Chemosphere 244:125491
Wazir MB, Daud M, Ali F, Al-Harthi MA (2020) Dendrimer assisted dye-removal: a critical review of adsorption and catalytic degradation for wastewater treatment. J Mol Liq 315:113775
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This manuscript has not been published or presented elsewhere in part or in its entirety.
Consent for publication
All authors agreed to publish this article in the International Journal of Environmental Science and Technology.
Additional information
Editorial responsibility: Q. Aguilar-Virgen.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jafarian, E., Hekmatiyan, A., Cheraghdar, A. et al. Elimination performance of Nile blue from wastewater using by carboxymethyl cellulose-graft-poly(methacrylic acid-co-acrylamide)/kaolin nanocomposite hydrogel. Int. J. Environ. Sci. Technol. 20, 9933–9944 (2023). https://doi.org/10.1007/s13762-023-05096-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-05096-0