Abstract
Taking a circular approach to mining facilities requires the further exploitation of produced solid wastes, which are now considered as potential raw materials. This research aims to the re-utilization of specific mining wastes, containing mainly geologically degraded serpentinized minerals, produced during the minerals’ enrichment process of extractive magnesite industry, combined with the addition of chromite ore, aiming to the upgrading of refractory properties of the product, by applying the appropriate thermal treatment. A representative sample examined, corresponding to the proper blending of different mineral waste samples from several waste piles of mining area, combined with various chromite ore’s content, followed by the investigation of optimum thermal treatment, considering the applied temperature and time. The scope was to maximize the (desired) forsterite mineral phase in the product and, hence, to improve its refractory properties. The optimum results (e.g., considering the firing shrinkage level and the mechanical strength) achieved by the application of thermal treatment at 1300 °C and after heating time for 120–240 min. The refractory properties generally improved after mixing of examined mining wastes and chromite ore, due to the achievement of the best molar ratio of constituents [MgO]/[SiO2] = 2.2, regarding the additive, enhancing the formation of forsterite, whereas the application of heating temperatures over 1300 °C led to the melting of enstatite mineral phase, resulting to the degradation of product. The obtained results reveal that the produced sintered products can exhibit better refractory properties, and can be used as refractory raw materials for relevant applications up to 1300 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The produced solid wastes, deposited after the magnesite mineral recovery from the respective ores, in the "Grecian Magnesite SA" (Northern Greece) open mining site, are currently estimated to be more than 35 × 106 tons in total. The possible valorization and further exploitation of these mining wastes, consisting mainly from degraded serpentinized peridotites minerals, is of particular significance, due to the potential strong economic perspectives, considering the application of circular economy aspects, as applied in the mining sector (Kalaitzidou et al. 2023). This approach, if successful, may be also applied to relevant mining industries worldwide, by the introduction of important (near) zero-waste concept (Pagona et al. 2022a, b; Kalaitzidou et al. 2022a, b; Pagona et al. 2021, 2020; Tzamos et al. 2020).
The production of serpentine mining wastes produced in Greece is approximately 4,350 kg/per capita throughout a year and the global production of relevant wastes results in more than 0.54 million tons, while the amount of serpentine deposits is estimated at hundreds of millions of tons, spread worldwide (Kalaitzidou et al. 2023; Carmignano et al. 2020). Even though there are several applications for serpentine minerals, the respective wastes present currently no actual commercial value, setting the need for reversing the serpentinization (geological degradation) process, since the expected new product from the reverse process (consisting mainly from the forsterite mineral phase) is actually an added-value product. However, the exploitation of high volumes of serpentine wastes not only offers economic advantages, but also ameliorates the landscape of the mine area, since the respective wastes will no longer be stock-pilled.
The serpentine group of minerals is composed mainly from chrysotile, lizardite, and antigorite, and their formation is the result of olivine’s (or of other relevant raw materials) reaction with water, containing relatively high quantities of magnesium and silicates (Hrsak et al. 2005). When the serpentinite minerals are thermally treated above 800 °C, then their structural water is released, leading approximately to 12% reduction of volume, and favoring the subsequent crystallization of produced amorphous mass toward the forsterite (Mg2SiO4) and enstatite (Mg2Si2O6) mineral phases (Zulumyan et al. 2018; Cheng et al. 2002). Therefore, the reverse process of geological serpentinization, as induced by current technological innovations, can result to the re-formation of desired forsterite, aiming to the enhancement of limited refractory properties in the raw serpentine ores; however, this procedure requires the presence of sufficient magnesite content (Pagona et al. 2022b, 2020). Noting also that several other additives may be also applied to induce the transformation of higher melting orthosilicate mineral phase to the lower melting metasilicate phase (Cheng et al. 2002).
Chromite ore (CO) is a material of low cost, being rather abundant in Greece, and presenting interesting properties, such as high melting point, average thermal expansion, neutral chemical behavior, and relatively high resistance in corrosion; therefore, it can be considered as potential additive/material that can result to the desirable process of olivine mineral phase formation, which is mainly containing from the required forsterite, and hence, to the respective refractory upgrade of product (Baklavaridis et al. 2021; Mcewan et al. 2011; Nemat et al. 2016). The chromite ore consists mainly from chromium spinel, which may contain in different proportions chromium, magnesium, aluminum, and iron and is a common commercial raw material, used also for the production of refractories (Bhandary et al. 2016). The addition of Cr2O3 to the mixture of forsterite refractory materials was also reported to increase the Mg–Al–Cr spinel content in the final product, showing a superior quality product (Tang et al. 2021; Sarkar et al. 2002). According to Jollands et al. (2018), the Cr3+ diffusion into the forsterite structure is highly dependent on its initial concentration.
Following the previously published promising results of Pagona et al. (2021), this study aims to the further examination of chromite ore (CO) role, leading to the reverse serpentinization process. The used CO originates from the deposits of Jurassic ophiolite minerals in North-Western Greece (Vourinos mountain), consisting mostly from the chrome ore-bearing dunite. This study examines the potential of CO utilization in combination with the magnesite mining (serpentinized) wastes after the application of optimum thermal treatment, expecting to result in the transformation of stocked mining wastes (considered rather as by-products) from the "Grecian Magnesite SA" mining area toward the production of commercial refractory materials.
This combination of solid wastes from the magnesite mine with the chromite ore deposits of Greece is considered an issue of particular environmental and economic importance. The chemical and mineralogical characterization of serpentinized solid wastes/by-products was already tested and presented in previous publications (Pagona et al. 2020; Tzamos et al. 2020), resulting to the selection of most representative samples, being subsequently blended/mixed to produce the specific sample examined in this study. Then, the detailed chemical and mineralogical characterization of composite mineral sample performed with and without the presence of examined additive (CO), regarding its effect to specific physico-chemical properties of raw serpentine waste, and specifically its effect for the enhancement of refractory properties for the thermally produced materials at different high temperatures (in the range 850–1600 °C). This range selected to examine the effect of respective temperatures on the induction and production of new stable and crystallized mineral phases, as quantified by XRD analysis and aiming for a greater added value refractory product.
Material and methods
The chemical analysis, regarding the elemental content of examined mineral samples, i.e., CO and composite mining waste sample, was initially performed. The samples pulverized by using a laboratory ball mill down to < 73 μm size. The digestion of 0.2 g of pulverized composite mineral sample took place in Teflon vials placed in a sand bath, until incipient dryness by the addition of 1 mL of concentrated HClO4 and 15 mL of concentrated HF. Moreover, 20 mL of 6 N HCl was added for the re-dissolution of the produced residue, which was ultimately diluted to 200 mL with the addition of ultra-pure water. Flame Atomic Absorption Spectroscopy (FAAS, PerkinElmer Atomic Absorption Spectrometer PinAAcle 500) was used for the chemical analysis of this sample, while the chemical composition of CO was determined by quantitative XRF, conducted by the Bureau Veritas Minerals Pty Ltd.
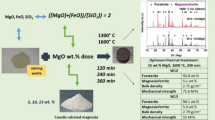
Then, the mining composite waste sample mixed and homogenized with the addition of different CO quantities, using ratios: 5, 10, 15, 20, 40, and 60 wt.%. The mixed samples pelletized and thermally treated by using the programmable control electric furnace (type SNOL 6.7/1300 LSC01) for heating at the maximum temperature 1300 °C (heating rate of 6.5 °C/min) and by Thermowatt (model TGH30L 380 V 10KW) for heating at temperatures higher than 1300 °C (heating rate of 10 °C/min). For the selection of optimum thermal treatment the examined temperatures and duration were 850 °C for 60, 120, 240, and 360 min, 1300 °C for 30, 60, 120, 240, and 360 min, 1400 °C for 120 min and 1600 °C for 120 min, and afterward, the samples rest at room temperature to cool.
Table 1 summarizes the chemical composition of the composite sample, of examined additive (CO), as well as of the blended samples. It is noticeable that the relatively high MgO concentration presence in CO increases, respectively, the MgO/SiO2 ratio of blended samples, according to the higher CO addition, i.e., 1.35, 2.67, 3.98, 5.30, 10.57, and 15.84%. For the determination of the loss on ignition (LOI) the samples fired at 950 °C by using the electric furnace SNOL.
For the evaluation of refractory behavior, regarding the blended and after the thermal treated products, their apparent porosity (AP%), bulk density (BD), and water absorption (WA%) properties were measured, according to the boiling water method (ASTM C20) (ASTM 2017). Furthermore, the determination of firing shrinkage (FS%) was estimated by using a calibrated caliper along the diameter and using the (D0-D1)/D0) ratio, i.e., the subscripts 0 and 1 correspond to the sample dimensions prior and after the applied thermal treatment. Moreover, surfaces images of the samples received by the polarizing optical microscope (POM), acquired with a ZEISS-POM instrument, which includes a polarizer slit with 20 × resolution. A Carver laboratory press utilized for the mechanical strength tests.
After the application of thermal treatment, the products pulverized and their mineralogical characterization followed. In order to determine the structural and mineralogical characteristics of fired products the X-ray diffraction (XRD) measurements conducted by using the Brüker D8 Advance diffractometer (powder XRD). The data provided by these measurements were derived at 2θ from 5° to 70°, scan time 0.2 s, and an increment of 0.02, while a filament X-ray tube of Cu with a wavelength 1.5418 Å and the detector LYNXEYE (1D mode) was applied. The data analysis software used for the qualitative and quantitative analysis of XRD patterns was the JADE XRD.
Results and discussion
Microscopic study
The evaluation of microstructure by the obtained micrographs from optical microscope took place, regarding the thermally treated products after their subsequent cooling, in order to estimate initially the surface (and get an idea of the refractoriness) of blended samples. Selected representative images are presented in Fig. 1, corresponding to the thermal treatment at heating temperature 1300 °C (for 120-min heating time) of the composite sample, as well as with the addition of 10 or 60 wt.% CO (i.e., under the respective “boundary” concentration conditions), and for the thermal treatment at heating temperature 1600 °C (for 120-min heating time) of the composite sample with the addition of 10 wt.% CO.
Optical microscope images of (a) initial composite sample, b with the addition of 10 wt.% CO, after firing at 850 °C for 120 min, c initial composite sample, d with the addition of 10 wt.% CO, e with the addition of 60 wt.% CO, after firing at 1300 °C for 120 min, and f with the addition of 10 wt.% CO after firing at 1600 °C for 120 min
It is noticeable that an alteration of surface takes place by the addition of CO in the thermally treated blended products, in agreement with the results previously shown by Kalaitzidou et al., (2022a) and Pagona et al. (2021). Due to the applied thermal treatment (Fig. 1, but also Figure S1 and Figure S2 of supplementary material) the resulted granular content of these samples presents locally different compositions. The surface of products is highly affected by the increase of temperature with the minimum alteration exhibited for the thermal treatment at 850 °C.
Furthermore, the white and black particles on the surface of respective product are increasingly reported, when the CO is added in the blended samples in higher quantities, marked in Fig. 1 as red and blue circles, respectively. This increase is more likely owed to the fact that different crystalline phases are created in the products. The white glassy phase that appears on the surfaces of products (as red circles) can be attributed to protoenstatite formation, which is a result of enstatite re-crystallization, occurring due to the presence of SiO2 that is free to react with the MgO, disintegrated from the MgCO3 at higher firing temperatures than 1300 °C. Overall, this procedure finally results to the formation of (undesirable) higher serpentine amount in the thermally treated products, leading to the degradation of required refractory properties. At the same time, the formation of spinel causes the black phases spotted on the surface of thermally treated products (denoted as blue circles).
It is quite clear that the comparison of size granules, as shown in Figs. 1b, 1d, and Fig. 1f, respectively, shown in the surfaces of products after thermal treatments at firing temperatures 850 °C, 1300 °C, and 1600 °C, is larger at the thermal process at 1600 °C, than those at obtained at lower temperatures.
In addition, it appears that the sintering of mineral particles was not achieved (denoted as green circle), even after firing at 1600 °C. This can be attributed to the observed higher presence of channels at the borders of granules and the formation of cracks, along with the increase of glassy phases in the thermally treated product (shown by the black arrow in Fig. 1). The increase of glassy phases in the product after thermal treatment at 1600 °C results to its deformation, due to liquidification; therefore, after the application of consecutive firing applications this product is expected to be quickly collapsed/destroyed. The thermal treatments of composite sample at 1300 °C for 30 min showed that this heating time was insufficient, unlike the better results derived for the thermal treatment at 1300 °C for 120 min. In addition, when comparing the thermal treatment at 1400 °C to that at 1600 °C (for the same duration of 120 min), the effect of heating temperature is better shown for the products that treated thermally at 1600 °C for 120 min. The CO/composite samples examined only for research comparison purposes and were thermally treated only at the optimum temperatures of 1300 °C and 1600 °C for 120 min. The addition of 10 wt.% CO to the composite sample resulted to higher forsterite content and mechanical strength at 1300 °C and 1600 °C (for 120 min). The higher percentages (20, 40, and 60% wt.%) of CO additions to this sample are not considered practically applicable, due to the increased percentage of Al2O3 (see also Table 1), which increases the glassy phase and causes the reduction of mechanical strength.
Firing shrinkage
The effects of CO addition on the firing shrinkage (FS%) parameter, being among the most significant properties considered for the refractory index of materials, are shown in Fig. 2. The respective data indicate that when the heating temperature is increased from 850° to 1300 °C, rather notable increase of firing shrinkage can be observed, not only for the original composite sample (from 1.1 up to 6.7%), but also for the products obtained after CO addition, e.g., with 10% wt. CO addition the firing shrinkage increased from 1.7 up to 5.9%. As the temperature of thermal treatment increased from 1300 °C to 1600 °C, all the examined specimens began to be densified and amorphous glass phases are formed (as observed from the increase of channels around the granules, see also Fig. 1d), which is also shown in the results of firing shrinkage parameter, e.g., with 10 wt.% of CO addition the firing shrinkage increased from 1.7 up to 13.4%; this is a significant disadvantage for potential refractory materials. The trend for the firing shrinkage results, regarding the other CO examined ratios, is similar to that of 10 wt.% CO addition. This is attributed to the maximization of forsterite formation at temperatures above 1000 °C and the simultaneous melting of enstatite (Grammatikakis et al. 2019; Nemat et al. 2019). Moreover, the loss of significant amount of structural hydroxyl groups and of water can led to the decrease of product volume (Mymrin et al. 2020). The addition of CO in combination with the thermal treatment at 850 °C results to the slight increase of firing shrinkage, as compared with the same sample but without the presence of additive. However, at even higher temperatures (i.e., 1300 °C) the results are reversed, showing that the addition of chromite ore can reduce the firing shrinkage, thus improving the refractory quality of the product, which should be generally retained at ≤ 5% range (considered as the upper permissible limit). The chromite mineral presents an almost linear expansion of about 1.3% at 1400 °C; therefore, when added to magnesia-containing minerals, as in this case, it is expected to increase the thermal shock resistance of produced refractory material and results also in higher compressibility (Mcewan et al. 2011; Wang et al. 2012). The optimum results, as reported for the maximum addition of 60 wt.% CO, can be attributed to the reduced LOI in the product, noting however that this high CO addition was examined only for research/comparison needs, as it is not practically applicable.
Water absorption–apparent porosity–bulk density
For a refractory product (e.g., brick) the water absorption (WA%), the apparent porosity (AP%), and the bulk density (BD) are considered as typical and important indicative parameters, regarding its quality. The relevant industry aims to the production of low porosity and high-density refractory products, which are resistant to any chemical attacks during the application of high operating temperatures, along with bulk density values greater than 2.6 g/cm3, being linked to lower porosity; these products are considered to present superior quality properties (Surendranathan 2014). The WA%, the AP%, and the BD (g/cm3) results of examined fired samples without and with the presence of CO (examined additions: 10, 20, 40, and 60 wt.%), when the sample is thermally treated at 1300 °C and at 1600 °C (for the same duration of 120 min) are presented in Fig. 3.
It is obvious that all these parameters appeared to be smaller, after the application of thermal treatment at 1600 °C, when compared to the respective treatment at 1300 °C (both for 120 min), due to increased densification and increase of glassy phases presence that can fill the existing inter-granular voids, pores, and cavities, and hence, resulting to denser material (Liu et al. 2021; Zargar et al. 2012). Moreover, for both thermal treatments the CO addition up to 20 wt% increases the WA% and AP% parameters, whereas for even higher CO additions (i.e., > 20 wt.%) the respective values show a plateau for the thermal treatment at 1300 °C and a decrease for the thermal treatment at 1600 °C. More specifically, after the application of thermal treatment at 1300 °C for 120 min, the WA (%) and AP (%) increased from 6.10 (for the initial composite sample) to 7.64 (for 20% CO addition) and from 16.70 (initial sample) to 19.61 (20% CO), respectively. For greater than 20 wt. % CO addition the WA% values resulted in a plateau and for the respected thermal treatment the AP% measured values are 20.26% and 20.78% for 40 and 60 wt.% CO additions. When the samples treated thermally at 1600 °C and 120 min, the WA% parameter dropped from 1.36% (initial sample) to 1.21% (with 60 wt.% CO) and the AP% parameter decreased from 3.41% (initial sample) to 3.12 (with 60 wt.% CO), respectively.
The BD values (g/cm3) for both thermal treatments show a decrease with the addition of CO up to 20 wt.%, whereas by the further increase of CO addition (> 20 wt.%), this parameter also increases. This can be attributed to the higher percentages of spinel formation, as verified also from the XRD quantification (Table 2). More specifically, for the thermal treatment at 1300 °C (for 120 min), the BD parameter decreased from 2.74 g/cm3 (original sample) to 2.57 g/cm3 (with 20 wt.% CO addition) and, afterward, increased to 2.69 g/cm3 (with 60 wt.% CO). The same trend is observed for the products of thermal treatment at 1600 °C (for 120 min); the BD slightly dropped from 2.51 g/cm3 (initial sample) to 2.49 g/cm3 (with 20 wt.% CO) and, afterward, increased to 2.58 g/cm3 (with 60 wt.% CO).
Mechanical strength
The mechanical strength is a property of high importance for refractory materials, since it provides information on the durability and stability of material during its application in high temperatures. In Fig. 4 the respective data from the mechanical strength tests are presented, showing that the compressive strength measurements of thermally treated products present the highest values for the products treated at temperature 1300 °C, with the optimum results obtained for heating times 120–240 min. The reported values are greater than 90 MPa, i.e., in the same magnitude as those reported by Tang et al. (2021).
The mechanical strength values also indicate that the blended samples with the addition of CO can increase the mechanical strength of products with the respective results to be further increased with the addition of 60 wt.% CO, which however cannot be considered as practically applicable. The non-proportional results, corresponding to the thermal treatment at 1300 °C for 120 min and for the 15–60 wt% CO addition, can be attributed to the impurities of used raw materials and more specifically, to the ratios of Cr2O3/FeO and of Cr2O3/Al2O3 oxides that are competing for the spinel formation. The increase of Al2O3 induces the formation of glassy phases at higher percentages, which results in the lowering of the product's mechanical strength, due to the formation of Al spinel, and causes overall the deterioration of products’ mechanical strength (Pagona et al. 2021). The application of thermal processes at temperatures higher than 1300 °C induces the formation of glassy phases at higher percentages (Figure S2 at supplementary materials), which decreases the strength of bonds between the grain boundaries, hence causing lower mechanical strength (< 22 MPa). The addition of CO in higher percentages (i.e., between 20 and 60 wt.%) is not generally considered as practically applicable, due to the increased co-presence of Al2O3 (Table 1), which results to the increase of glassy phases and the decrease of mechanical strength and, therefore, the respective products are not considered as suitable for refractory materials (Hu et al. 2018). The results reveal that the addition of 10 wt.% CO shows the highest values obtained for the mechanical strength parameter, due to the lower Al2O3 content, when compared to the higher CO additions.
XRD analysis of products after the application of thermal treatment
The patterns from the XRD analysis of representative products are shown in Fig. 4, considering the applied thermal treatment (for heating time 120 min) and for the initial composite sample with and without the addition of 10 wt.% CO. Moreover, the respective mineralogical content (wt.%) for all these products is presented in Table 2, corresponding to the mean percentage content for the fired samples, while the detailed XRD patterns are shown in the Supplementary Material (as Figures S3-S12). Comparing these XRD patterns with those in the JCPDS database, the major crystal phase in all examined products, is forsterite (PDF#88-0999), belonging in the olivine group of minerals, with the presence of different enstatite phases, i.e., enstatite (PDF#84-2027), ferroan enstatite (PDF#88-1911), protoenstatite (PDF#76-1806), and clinoenstatite (PDF#84-0652), belonging to the pyroxene group of minerals, and spinel, corresponding to the composition of Mg(Cr,Fe,Al)2O4 different classes of spinels (i.e., magnesio-ferrite: PDF#88-1935 and magnesio-chromite: PDF#87-1175). Mielcarek et al. (2004) studied the polymorphism of magnesium silicate, reporting that the heating temperature results in the crystallization of protoenstatite at temperatures higher than 1250 °C and up to 1420 °C, where this mineral phase is completely formed, whereas at even higher temperatures (above 1420 °C) the protoenstatite crystallites become unstable and finally, after cooling they inverse to clinoenstatite crystallites. These results reveal also that the increase of heating time does not affect significantly the pyroxenes’ content, for all the examined heating temperatures. However, some differences in the mineralogical content have been observed with the application of the highest examined heating temperature (1600 °C), presenting the highest forsterite percentage in the treated sample, since the enstatite content is eliminated, due to its melting point being at 1557 °C (Nemat et al. 2019).
Although the increase of heating temperature generally results to the (desired) increase of forsterite content in the product, however, the over-heating temperature can lead to the sintering of samples’ granules (above the temperature of 1300 °C), caused by the lower melting points of co-existing impurity cations, such as Ca2+, Fe2+/Fe3+, Al3+, resulting to the slight decline of refractory properties (Wang et al. 2017). Furthermore, the re-crystallization of free MgO and free silica that occurs at the heating temperature of 1300 °C is mainly attributed to the development of forsterite and enstatite mineral phases and the subsequent increase of pyroxene content (Cheng et al. 2002). The formation of spinels is reported for the applied thermal treatments but for temperatures over 1300 °C (Table 2), since the magnesio-chromite formation requires higher temperatures than 1250 °C (Brigida et al. 2007). As already observed from the data of Table 1, the addition of CO found to increase the MgO/SiO2 ratio content in the blended samples, which in turn can favor the formation of (desirable) forsterite mineral phase. However, the addition of CO at higher percentages than 20 wt% in the initial (composite) sample shows a parallel increase of spinel formation that results in the reduction of forsterite content, due to the increase of impurities content, which in turn favors the increase of glassy phase’s formation. As already reported, according to Jollands et al. (2018), the diffusion of Cr3+ in the structure of forsterite is highly dependent upon its concentration, while the co-existence of enstatite affects the formation of magnesio-chromite (Fig. 5).
The efficient conversion of serpentinized waste/by-products of the studied magnesite mine toward the formation of new valuable products/uses, showing better refractory properties, can extend the life of respective mine by reducing the useful mineral extraction requirements and by using in the production processes new sources of alternative materials (i.e., instead of imported dunite, the so-produced forsterite), thus applying by this way the main principal of circular economy. Furthermore, the process develops feasible exploitation options at the end of materials’ life enrichment process and allows its reusability. The importance of sustainability lies in both aspects of keeping the resources in use for as long as possible and by limiting the final disposal of generated mining waste. Moreover, the reverse of the serpentinization process presents zero CO2 emissions, which is a significant environmental advantage for the development of the new process (Khorami et al., 2019; Upadhyay et al. 2021).
Conclusion
The relatively small addition of chromite ore (as an additive) to the industrial solid wastes, obtained from the magnesite mining during the enrichment processes, results in the amelioration of major refractory properties/parameters for the obtained products after thermal treatment, i.e., can improve the mechanical strength and decrease the firing shrinkage of the respective product, when a thermal treatment is applied at 1300 °C, with the optimum results being presented for the heating times 120–240 min. The increase of the (desired) forsterite content formation at 1300 °C, as revealed by the XRD patterns of the products, can be attributed to the increase of MgO/SiO2 ratio in the final products, considering the practical addition of 5–20 wt.% CO. However, the addition of higher CO percentages lead to the simultaneous increase of impurities (e.g., Fe oxides and Al2O3) and appear that the formation of spinels dominates over the formation of forsterite; therefore, the refractory properties of WA and AP are negatively affected, while the BD value increases.
On the other hand, the increase of heating temperature above 1300 °C causes the formation of glassy phases in the product and provokes the degradation of refractory properties, making it unsuitable for relevant uses. It should be pointed out that the refractory materials produced after the application of firing processes up to 1300 °C present not only higher added value, but also represent an actual solution to overcome the potential environmental problem, caused by the deposition of several million tons of waste rocks. These results suggest that the materials produced from this study can be suitable for specific technological applications, such as the production of refractory materials (used up to 1300 °C), or forsterite-magnesiochromite composites, in the context of mining circular economy, closing the resource loop with the proper recycling and re-utilization of mineral resources/by-products.
References
ASTM (2017) ASTM C20-00 Standard Test Methods for Apparent Porosity, Water Absorption, Apparent Specific Gravity, and Bulk Density of Burned Refractory Brick and Shapes by Boiling Water. ASTM Int
Baklavaridis A, Vatalis K, Karayannis V, Benetis PN, Charalampides G (2021) Mineralogical characterization and evaluation of chromite ore in grevena and kozani vourinos massif, Western Macedonia, Greece. Min Miner Depos 15:11–18. https://doi.org/10.33271/mining15.01.011
Bhandary AK, Gupta P, Mukherjee S, Chaudhuri MG, Dey R (2016) Beneficiation of low grade chromite ore and its characterization for the formation of magnesia-chromite refractory by economically viable process. In: World Academy of Science, Engineering and Technology International Journal of Chemical and Molecular Engineering, pp 1096–1104
Brigida C, Poli S, Valle M (2007) High-temperature phase relations and topological constraints in the quaternary system MgO-Al2O3-SiO2-Cr2O3: an experimental study. Am Mineral 92:735–747. https://doi.org/10.2138/am.2007.2327
Carmignano ORRD, Vieira SS, Brandão PRG, Bertoli AC, Lago RM (2020) Serpentinites: mineral structure, properties and technological applications. J Braz Chem Soc 31:2–14. https://doi.org/10.21577/0103-5053.20190215
Cheng TW, Ding YC, Chiu JP (2002) A study of synthetic forsterite refractory materials using waste serpentine cutting. Miner Eng 15:271–275. https://doi.org/10.1016/S0892-6875(02)00021-3
Grammatikakis EI, Kyriakidis E, Demadis D, Cabeza Diaz A, Leon-Reina L (2019) Mineralogical characterization and firing temperature delineation on minoan pottery, focusing on the application of micro-raman spectroscopy. Heritage 2:2652–2664
Hrsak D, Malina J, Hadzipasic A (2005) The decomposition of serpentine by thermal treatment. Mater Tehnol 39:225–227
Hu Y, Li Y, Lou J, He H, Zhang X (2018) Effects of sintering temperature and holding time on densification and mechanical properties of MIM HK30 stainless steel. Int J Metall Met Phys 3:8022
Jollands MC, O’Neill HSC, Van Orman J, Berry AJ, Hermann J, Newville M, Lanzirotti A (2018) Substitution and diffusion of Cr2+ and Cr3+ in synthetic forsterite and natural olivine at 1200–1500 °C and 1 bar. Geochim Cosmochim Acta 220:407–428. https://doi.org/10.1016/j.gca.2017.09.030
Kalaitzidou K, Pagona E, Stratigousis P, Ntampou X, Tzamos E, Zaspalis V, Zouboulis A, Mitrakas M (2022a) Hematite nanoparticles addition to serpentine/pyroxenes by-products of the “Grecian Magnesite SA” mine at Gerakini (Halkidiki) for the production of refractories submitted. Appl Sci 12:2094. https://doi.org/10.3390/app12042094
Kalaitzidou K, Pagona E, Zouboulis A, Mitrakas M (2022) Exploitation of the fine rejected run of mine (ROM 0–4 mm) material to produce refractories in combination with the mining by-products of magnesite mine. Mater Chem Phys 292:126743
Kalaitzidou K, Pagona E, Mitrakas M, Zouboulis A (2023) MagWasteVal project—towards sustainability of mining waste. Sustainability 15:1648. https://doi.org/10.3390/su15021648
Liu X, Zhou Y, Liu X, Li R, Li S, Li C (2021) SiC-based porous ceramic carriers for heat-conductive phase change materials through carbothermal reduction method. Int J Appl Ceram Technol 18:91–99. https://doi.org/10.1111/ijac.13639
Mcewan N, Courtney T, Parry RA, Knupfer P (2011) Chromite-a cost-effective refractory raw material for refractories in various metallurgical applications. South Afr Pyrometall 4:359–370
Mielcarek W, Nowak-Woźny D, Prociów K (2004) Correlation between MgSiO3 phases and mechanical durability of steatite ceramics. J Eur Ceram Soc 24:3817–3821. https://doi.org/10.1016/j.jeurceramsoc.2003.12.030
Mymrin V, Presotto P, Alekseev K, Avanci MA, Rolim PHB, Petukhov V, Taskin A, Gidarakos E, Valouma A, Yud G (2020) Application of hazardous serpentine rocks’ extraction wastes in composites with glass waste and clay-sand mix to produce environmentally clean construction materials. Constr Build Mater 234:117319
Nemat S, Ramezani A, Emami SM (2019) Recycling of waste serpentine for the production of forsterite refractories: the effects of various parameters on the sintering behavior. J Aust Ceram Soc 55:425–431. https://doi.org/10.1007/s41779-018-0250-z
Nemat S, Ramezani A, Emami SM (2016) Possible use of waste serpentine from Abdasht chromite mines into the refractory and ceramic industries. Ceram Int 42:18479–18483. https://doi.org/10.1016/j.ceramint.2016.08.184
Pagona E, Tzamos E, Grieco G, Zouboulis A, Mitrakas M (2020) Characterization and evaluation of magnesite ore mining by-products of Gerakini mines (Chalkidiki, N. Greece). Sci Total Environ 732:139279
Pagona Ε, Kalaitzidou K, Zouboulis A, Mitrakas M (2021) Effects of additives on the physical properties of magnesite ore mining by-products for the production of refractories. Miner Eng 174:107247
Pagona E, Kalaitzidou K, Zouboulis A, Mitrakas M (2022) Estimation and addition of MgO dose for upgrading the refractory characteristics of magnesite ore mining wastes/by-products", submitted to Waste Biomass Valorization. Science 5:441
Pagona E, Kalaitzidou K, Zaspalis V, Zouboulis A, Mitrakas M (2022b) Effects of MgO and Fe2O3 addition for upgrading the refractory characteristics of magnesite ore mining waste/by-products. Clean Technol 4:1103–1126. https://doi.org/10.3390/cleantechnol4040067
Sarkar R, Kumar Das S, Banerjee G (2002) Effect of addition of Cr2O3 on the properties of reaction sintered MgO-Al2O3 spinels. J Eur Ceram Soc 22:1243–1250. https://doi.org/10.1016/S0955-2219(01)00446-0
Surendranathan AO (2014) An introduction to ceramics and refractories. Science. https://doi.org/10.1201/b17811
Tang H, Peng Z, Gu F, Yang L, Tian W, Zhong Q, Rao M, Li G, Jiang T (2021) Chromium-promoted preparation of forsterite refractory materials from ferronickel slag by microwave sintering. Ceram Int 47:10809–10818. https://doi.org/10.1016/j.ceramint.2020.12.198
Tayebi-Khorami M, Edraki M, Corder G, Golev A (2019) Re-thinking mining waste through an integrative approach led by circular economy aspirations. Minerals 9:286. https://doi.org/10.3390/min9050286
Tzamos E, Bussolesi M, Grieco G, Marescotti P, Crispini L, Kasinos A, Storni N, Simeonidis K, Zouboulis A (2020) Mineralogy and geochemistry of ultramafic rocks from rachoni magnesite mine, Gerakini (Chalkidiki, Northern Greece). Minerals 10:934. https://doi.org/10.3390/min10110934
Upadhyay A, Laing T, Kumar V, Dora M (2021) Exploring barriers and drivers to the implementation of circular economy practices in the mining industry. Resour Policy 72:102037
Wang S, Liu X, Fei Y, He Q, Wang H (2012) In situ high-temperature powder X-ray diffraction study on the spinel solid solutions (Mg 1-xMn x)Cr 2O 4. Phys Chem Miner 39:189–198. https://doi.org/10.1007/s00269-011-0474-8
Wang WB, Shi ZM, Wang XG, Wang ZX, Cao Z, Fan W (2017) The synthesis and properties of high-quality forsterite ceramics using desert drift sands to replace traditional raw materials. J Ceram Soc Japan 125:88–94. https://doi.org/10.2109/jcersj2.16268
Zargar HR, Oprea C, Oprea G, Troczynski T (2012) The effect of nano-Cr2O3 on solid-solution assisted sintering of MgO refractories. Ceram Int 38:6235–6241. https://doi.org/10.1016/J.CERAMINT.2012.04.077
Zulumyan N, Isahakyan A, Beglaryan H, Melikyan S (2018) A study of thermal decomposition of antigorite from dunite and lizardite from peridotite. J Therm Anal Calorim 13:11201–1211
Acknowledgments
The authors wish to thank all who assisted in conducting this work.
Funding
Open access funding provided by HEAL-Link Greece. This research has been co‐financed by the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH – CREATE – INNOVATE (Grand Number: T1EDK-03543).
Author information
Authors and Affiliations
Contributions
CRediT roles: TE. conceptualized the study; KK., PE. curated the data; MM. acquired the funding and helped in methodology and validation; KK., SG. investigated the study; ZA. administrated the project; MM., ZA. supervised the study; TE. visualized the study; KK. helped in writing—original draft; TE., ZA., MM. contributed to writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Editorial responsibility: Samareh Mirkia.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kalaitzidou, K., Pagona, E., Skyfta, G. et al. Chromite ore addition to serpentinized magnesite mining wastes for the production of refractory products following thermal treatment. Int. J. Environ. Sci. Technol. 20, 13561–13570 (2023). https://doi.org/10.1007/s13762-023-04933-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-04933-6