Abstract
Aromatic triarylmethane dyes residues cause serious water pollution in environment. Adsorbents, such as graphene oxide (GO), have great potential for using them for removal of dyes such as crystal violet (CV) from wastewater. The GO was prepared from graphite-flakes (G) through chemical exfoliation method. The synthesized materials were characterized by the scanning electron microscopy, transmission electron microscopy, X-ray diffraction (XRD), ultraviolet–visible, and Fourier-transform infrared spectroscopies and then were tested their potential toward removal of the CV from aqueous solution via adsorption. Different parameters, i.e., adsorbent nature, adsorbent dose, appropriate temperature, contact time, and concentration of dye, were used to check the efficiency of adsorbents for the removal of the CV. Adsorption experimental results show that the removal capacity of dye for graphene oxide, graphite, and graphene quantum dots (GQDs) were recorded, i.e., 32.12, 33.48, and 34.46 mg/g, respectively, at T = 303 K with 0.1 g/L adsorbent dosage and 200 mg/L of adsorbate content for reaching efficient treatment time (60 min). The adsorption results show that the maximum removal efficiencies of the CV by the GO, the graphite (G), and the GQDs were recorded as: 94.86, 93.25, and 99.1%, respectively, for a 50 mg/L of dye solution. Moreover, the thermodynamic parameters show that the percent removal of dye and adsorption capacity in (mg/g) were enhanced with an incline in heating; this meant that the dye uptake for these adsorbents acted endothermic process. The Gibbs energy free (− ∆G), entropy (+ ∆S), and enthalpy (+ ∆H) values showed that the uptake/adsorption of CV by these adsorbents is spontaneous, endothermic, and entropy-driven in nature. The kinetic experiment shows that the adsorption data of CV dye on G, GO, and GQDs obey both the pseudo-first and pseudo-second-order adsorption mechanism. The adsorption on the prepared samples follows Langmuir and Freundlich isotherm models. Among these models the most fitted model was Langmuir adsorption isotherm and that the adsorption process is mostly performed by chemisorption with single coverage layer.
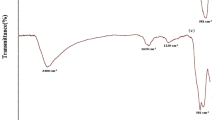
Graphical abstract













Similar content being viewed by others
References
Alam S, Khan MS, Umar A, Khattak R, Rahman NU, Zekker I, Bhowmick GD (2021a) Preparation of Pd–Ni nanoparticles supported on activated carbon for efficient removal of basic blue 3 from water. Water 13(9):1211
Alam S, Khan MS, Bibi W, Zekker I, Burlakovs J, Ghangrekar MM (2021b) Preparation of activated carbon from the wood of paulownia tomentosa as an efficient adsorbent for the removal of acid red 4 and methylene blue present in wastewater. Water 13(11):1453
Amin N, Abdelwahab O, El-Ashtoukhy E-S (2015) Removal of Cu(II) and Ni(II) by ion exchange resin in packed rotating cylinder. Desalin Water Treat 55(1):199–209
Anayurt RA, Sari A, Tuzen M (2009) Equilibrium, thermodynamic and kinetic studies on biosorption of Pb(II) and Cd(II) from aqueous solution by macrofungus (Lactarius scrobiculatus) biomass. Chem Eng J 151(1–3):255–261
Bacon M, Bradley SJ, Nann T (2014) Graphene quantum dots. Part Part Syst Charact 31(4):415–428
Di Sia P (2017) Nanotechnology among innovation, health and risks. Proc Soc Behav Sci 237:1076–1080
Elmorsi TM, Riyad YM, Mohamed ZH, Abd El Bary HM (2010) Decolorization of Mordant red 73 azo dye in water using H2O2/UV and photo-Fenton treatment. J Hazard Mater 174(1–3):352–358
Ersöz G (2014) Fenton-like oxidation of reactive black 5 using rice husk ash based catalyst. Appl Catal B 147:353–358
Fan X, Peng W, Li Y, Li X, Wang S, Zhang G, Zhang F (2008) Deoxygenation of exfoliated graphite oxide under alkaline conditions: a green route to graphene preparation. Adv Mater 20(23):4490–4493
Faniyi IO, Fasakin O, Olofinjana B, Adekunle AS, Oluwasusi TV, Eleruja MA, Ajayi EOB (2019) The comparative analyses of reduced graphene oxide (RGO) prepared via green, mild and chemical approaches. SN Appl Sci 1(10):1–7
Ghaedi M, Ansari A, Habibi M, Asghari A (2014) Removal of malachite green from aqueous solution by zinc oxide nanoparticle loaded on activated carbon: kinetics and isotherm study. J Ind Eng Chem 20(1):17–28
Ghaedi M, Mohammdi F, Ansari A (2015) Gold nanoparticles loaded on activated carbon as novel adsorbent for kinetic and isotherm studies of methyl orange and sunset yellow adsorption. J Dispersion Sci Technol 36(5):652–659
Hussain Z, Khan A, Sultan N, Ali M, Naz MY, Saed K, Sulaiman SA (2019) Thermo-chemical conversion of waste glass into non-vitreous porous material for adsorption application. J Mater Cycles Waste Manag 21(5):1132–1143
Jabeen S, Khan MS, Khattak R, Zekker I, Burlakovs J, Rubin S (2021) Palladium-supported zirconia-based catalytic degradation of rhodamine-b dye from wastewater. Water 13(11):1522
Kosynkin DV, Higginbotham AL, Sinitskii A, Lomeda JR, Dimiev A, Price BK, Tour JM (2009) Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 458(7240):872–876
Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, Muller RN (2008) Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev 108(6):2064–2110
Li H, Pan L, Nie C, Liu Y, Sun Z (2012) Reduced graphene oxide and activated carbon composites for capacitive deionization. J Mater Chem 22(31):15556–15561
Liu L, Zhang J, Tan Y, Jiang Y, Hu M, Li S, Zhai Q (2014) Rapid decolorization of anthraquinone and triphenylmethane dye using chloroperoxidase: catalytic mechanism, analysis of products and degradation route. Chem Eng J 244:9–18
Malik A, Khan A, Humayun M (2019) Preparation and chemical modification of rice husk char for the removal of a toxic dye (Orange G) from aqueous medium. Z Phys Chem 233(3):375–392
Malik A, Khan A, Anwar N, Naeem M (2020a) A comparative study of the adsorption of congo red dye on rice husk, rice husk char and chemically modified rice husk char from aqueous media. Bull Chem Soc Ethiop 34(1):41–54
Malik A, Khan A, Shah N, Khan MS (2020b) The kinetics and equilibrium thermodynamics study on the removal of direct blue and titan yellow dyes from aqueous media by modified rice husk char. Z Phys Chem 234(3):485–503
Rahman N, Ullah I, Alam S, Khan MS, Shah LA, Zekker I, Burlakovs J (2021) Activated Ailanthus altissima sawdust as adsorbent for removal of acid yellow 29 from wastewater: kinetics approach. Water 13(15):2136
Shaalan M, Saleh M, El-Mahdy M, El-Matbouli M (2016) Recent progress in applications of nanoparticles in fish medicine: a review. Nanomed Nanotechnol Biol Med 12(3):701–710
Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, Ruoff RS (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45(7):1558–1565
Sun Y, Wang S, Li C, Luo P, Tao L, Wei Y, Shi G (2013) Large scale preparation of graphene quantum dots from graphite with tunable fluorescence properties. Phys Chem Chem Phys 15(24):9907–9913
Tang L, Li X, Ji R, Teng KS, Tai G, Ye J, Lau SP (2012) Bottom-up synthesis of large-scale graphene oxide nanosheets. J Mater Chem 22(12):5676–5683
Umar A, Khan MS, Alam S, Zekker I, Burlakovs J, Rubin SS, Zahoor M (2021) Synthesis and characterization of Pd–Ni bimetallic nanoparticles as efficient adsorbent for the removal of acid orange 8 present in wastewater. Water 13(8):1095
Vickers NJ (2017) Animal communication: when i’m calling you, will you answer too? Curr Biol 27(14):R713–R715
Wang S, Zhang L, Xia Z, Roy A, Chang DW, Baek JB, Dai L (2012) BCN graphene as efficient metal-free electrocatalyst for the oxygen reduction reaction. Angew Chem Int Ed 51(17):4209–4212
Wang J, Yang N, Tang H, Dong Z, Jin Q, Yang M, Wang D (2013) Accurate control of multishelled Co3O4 hollow microspheres as high-performance anode materials in lithium-ion batteries. Angew Chem 125(25):6545–6548
Zhang X, Ma C, Wen K, Han R (2020) Adsorption of phosphate from aqueous solution by lanthanum modified macroporous chelating resin. Korean J Chem Eng 37(5):766–775
Acknowledgements
Abbas Khan and Saba Noor are grateful to Dr. Sayyar Ali Shah for his helpful role during the characterization of materials. The paper was supported of Higher Education Commission (HEC), Pakistan.
Author information
Authors and Affiliations
Contributions
AK helped in conceptualization, supervision, writing—reviewing and editing. SN contributed to data curation, writing—original draft preparation. MSK performed visualization, investigation. RK was involved in methodology, software. AM helped in adsorption experiments. UUR contributed to software, validation. LAS reviewed and edited the revision. NUR helped in software, validation (during revision). IZ was involved in writing—reviewing and editing.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Editorial responsibility: Samareh Mirkia.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khan, A., Noor, S., Khan, M.S. et al. Removal of crystal violet from wastewater using synthesized graphene quantum dots as adsorbents: kinetic approach. Int. J. Environ. Sci. Technol. 20, 13219–13232 (2023). https://doi.org/10.1007/s13762-023-04881-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-04881-1