Abstract
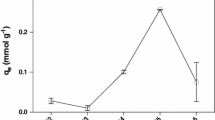
This study used four different macroalgae (Costaria costata, Hizikia fusiformis, Gracilaria verrucosa, and Codium fragile) as biosorbents to remove the heavy metals, Cd2+, Cu2+, Ni2+, and Pb2+. Among the studied macroalgae, Costaria costata showed the best biosorbent activity for heavy metal removal. A Fourier transform infrared spectroscopy analysis indicated that the cell wall structure of Costaria costata has various functional groups such as amine, sulfonate, negatively charged hydroxyl groups, and carboxyl groups. The modification of Costaria costata using HCl and NaOH was ineffective in terms of improving its biosorption capacity. The kinetic experiments and modeling data showed reaction ratios in the following descending order: Ni2+ > Cd2+ > Cu2+ > Pb2+. Additionally, the Freundlich model was able to describe the equilibrium data well except for Pb2+. The maximum heavy metal adsorption capacity for Pb2+ was 160.990 mg/g; this was higher than the other heavy metals: 45.094, 44.768, and 42.082 mg/g for Cu2+, Cd2+, and Ni2+, respectively. This means that the adsorption of heavy metals onto Costaria costata occurred via covalent bonding as opposed to electrostatic attraction. Moreover, metal biosorption by Costaria costata enhanced significantly as the initial solution pH increased from 2 to 4. Among the competing cations, Al3+ demonstrated the greatest inhibitory effect on heavy metal removal because of its high charge valence and affinity. Consequently, the dried biomass of Costaria costata could be used to potentially eliminate heavy metals from aqueous solutions due to its characteristic high efficiency, easy acquisition, low cost, and easy preparation.








Similar content being viewed by others
Availability of data and material
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Code availability
Not applicable.
References
Abdullah N, Yusof N, Lau WJ, Jaafar J, Ismail AF (2019) Recent trends of heavy metal removal from water/wastewater by membrane technologies. J Ind Eng Chem 76:17–38
Apiratikul R, Pavasant P (2008) Batch and column studies of biosorption of heavy metals by Caulerpa lentillifera. Bioresour Technol 99(8):2766–2777
Apiratikul R, Marhaba TF, Wattanachira S, Pavasant P (2004) Biosorption of binary mixtures of heavy metals by green macro alga Caulerpa Lentillifera. Songklanakarin J Sci Technol 26(2):199–207
Aung KT, Hong SH, Park SJ, Lee CG (2020) Removal of Cu(II) from aqueous solutions using amine-doped polyacrylonitrile fibers. Appl Sci 10(5):1738
Blanchard G, Maunaye M, Martin G (1984) Removal of heavy metals from waters by means of natural zeolites. Water Res 18(12):1501–1507
Çetinkaya Dönmez G, Aksu Z, Öztürk A, Kutsal T (1999) A comparative study on heavy metal biosorption characteristics of some algae. Process Biochem 34(9):885–892
Chen YG, Ye WM, Yang XM, Deng FY, He Y (2011) Effect of contact time, pH, and ionic strength on Cd(II) adsorption from aqueous solution onto bentonite from Gaomiaozi, China. Environ Earth Sci 64(2):329–336
Cheng SY, Show PL, Lau BF, Chang JS, Ling TC (2019) New prospects for modified algae in heavy metal adsorption. Trends Biotechnol 37(11):1255–1268
Choi EY, Hwang HJ, Kim IH, Nam TJ (2009) Protective effects of a polysaccharide from Hizikia fusiformis against ethanol toxicity in rats. Food Chem Toxicol 47(1):134–139
Choi HG, Kim YS, Kim JH, Lee SJ, Park EJ, Ryu J, Nam KW (2007) Effects of temperature and salinity on the growth of Gracilaria verrucosa and G. chorda, with the potential for mariculture in Korea. Springer, Dordrecht, pp 43–51
Coates J (2006) Interpretation of infrared spectra, a practical approach. In: Encyclopedia of analytical chemistry. Wiley, New York
Davis TA, Volesky B, Mucci A (2003) A review of the biochemistry of heavy metal biosorption by brown algae. Water Res 37(18):4311–4330
Deniz F, Karabulut A (2017) Biosorption of heavy metal ions by chemically modified biomass of coastal seaweed community: studies on phycoremediation system modeling and design. Ecol Eng 106:101–108
Du J, Zhang B, Li J, Lai B (2020) Decontamination of heavy metal complexes by advanced oxidation processes: a review. Chin Chem Lett 31(10):2575–2582
El-Sayed GO, Dessouki HA, Ibrahiem SS (2011) Removal of Zn (II), Cd (II) and Mn (II) from aqueous solutions by adsorption on maize stalks. Malaysian J Anal Sci 15(1):8–21
Ermakova S, Sokolova R, Kim SM, Um BH, Isakov V, Zvyagintseva T (2011) Fucoidans from brown seaweeds Sargassum hornery, Eclonia cava, Costaria costata: structural characteristics and anticancer activity. Appl Biochem Biotechnol 164(6):841–850
Fabre E, Dias M, Costa M, Henriques B, Vale C, Lopes CB, Pinheiro-Torres J, Silva CM, Pereira E (2020) Negligible effect of potentially toxic elements and rare earth elements on mercury removal from contaminated waters by green, brown and red living marine macroalgae. Sci Total Environ 724:138133
Feng NC, Guo XY (2012) Characterization of adsorptive capacity and mechanisms on adsorption of copper, lead and zinc by modified orange peel. Trans Nonferrous Met Soc China 22(5):1224–1231
Ferrer MRR, Kang JK, Choi JW, Lee CG, Park SJ (2021) Surface modification of activated carbon via HCl or NH4OH treatment to enhance the removal of Cr(VI) from aqueous solution. Desalin Water Treat 220:221–231
Figueira MM, Volesky B, Ciminelli VST, Roddick FA (2000) Biosorption of metals in brown seaweed biomass. Water Res 34(1):196–204
Gajda I, Stinchcombe A, Greenman J, Melhuish C, Ieropoulos I (2017) Microbial fuel cell—a novel self-powered wastewater electrolyser for electrocoagulation of heavy metals. Int J Hydrogen Energ 42(3):1813–1819
Goher ME, Abd El-Monem AM, Abdel-Satar AM, Ali MH, Hussian AEM, Napiórkowska-Krzebietke A (2016) Biosorption of some toxic metals from aqueous solution using non-living algal cells of Chlorella vulgaris. J Elem 21(3):703–714
Hong SH, Shin MC, Lee J, Lee CG, Song DS, Um BH, Park SJ (2021b) Recycling of bottom ash derived from combustion of cattle manure and its adsorption behaviors for Cd(II), Cu(II), Pb(II), and Ni(II). Environ Sci Pollut Res Int 28(12):14957–14968
Hong SH, Lee CG, Park SJ (2021a) Removal of Cd2+, Cu2+, Pb2+, and Ni2+ by sludge produced from liquid crystal display glass substrate. Int J Environ Sci Technol In press.
Hughes MN, Poole RK (1989) Metals and microorganisms. Springer, Berlin
Hwang EK, Baek JM, Park CS (2008) Cultivation of the green alga, Codium fragile (Suringar) Hariot, by artificial seed production in Korea. J Appl Phycol 20(5):469–475
Ibrahim WM (2011) Biosorption of heavy metal ions from aqueous solution by red macroalgae. J Hazard Mater 192(3):1827–1835
Ibrahim WM, Hassan AF, Azab YA (2016) Biosorption of toxic heavy metals from aqueous solution by Ulva lactuca activated carbon. Egypt J Basic Appl Sci 3(3):241–249
Jaafari J, Yaghmaeian K (2019) Optimization of heavy metal biosorption onto freshwater algae (Chlorella coloniales) using response surface methodology (RSM). Chemosphere 217:447–455
Javadian H, Ahmadi M, Ghiasvand M, Kahrizi S, Katal R (2013) Removal of Cr(VI) by modified brown algae Sargassum bevanom from aqueous solution and industrial wastewater. J Taiwan Inst Chem E 44(6):977–989
Kalyani S, Srinivasa Rao P, Krishnaiah A (2004) Removal of nickel (II) from aqueous solutions using marine macroalgae as the sorbing biomass. Chemosphere 57(9):1225–1229
Kang K, Lee C-G, Choi JW, Kim YK, Park SJ (2016) Evaluation of the use of sea sand, crushed concrete, and bentonite to stabilize trace metals and to interrupt their release from contaminated marine sediments. Water Air Soil Pollut 227(9):308
Lagergren SK (1898) About the theory of so-called adsorption of soluble substances. Sven Vetenskapsakad Handingarl 24:1–39
Lee CG, Alvarez PJJ, Nam A, Park SJ, Do T, Choi US, Lee SH (2017) Arsenic(V) removal using an amine-doped acrylic ion exchange fiber: Kinetic, equilibrium, and regeneration studies. J Hazard Mater 325:223–229
Leusch A, Holan ZR, Volesky B (1995) Biosorption of heavy metals (Cd, Cu, Ni, Pb, Zn) by chemically-reinforced biomass of marine algae. J Chem Technol Biotechnol 62(3):279–288
Lyonga FN, Hong SH, Cho EJ, Kang JK, Lee CG, Park SJ (2020) As(III) adsorption onto Fe-impregnated food waste biochar: experimental investigation, modeling, and optimization using response surface methodology. Environ Geochem Health 43:3303–3321
Mazur LP, Cechinel MAP, de Souza SMAGU, Boaventura RAR, Vilar VJP (2018) Brown marine macroalgae as natural cation exchangers for toxic metal removal from industrial wastewaters: A review. J Environ Manag 223:215–253
McBride MB (1994) Environmental chemistry of soils. Oxford University Press, Oxford
Oves M, Khan MS, Zaidi A (2013) Biosorption of heavy metals by Bacillus thuringiensis strain OSM29 originating from industrial effluent contaminated north Indian soil. Saudi J Biol Sci 20(2):121–129
Prasher SO, Beaugeard M, Hawari J, Bera P, Patel RM, Kim SH (2004) Biosorption of heavy metals by red algae (Palmaria palmata). Environ Technol 25(10):1097–1106
Raize O, Argaman Y, Yannai S (2004) Mechanisms of biosorption of different heavy metals by brown marine macroalgae. Biotechnol Bioeng 87(4):451–458
Roginsky S, Zeldovich YB (1934) The catalytic oxidation of carbon monoxide on manganese dioxide. Acta Phys Chem 1(554):2019
Romera E, González F, Ballester A, Blázquez ML, Muñoz JA (2007) Comparative study of biosorption of heavy metals using different types of algae. Bioresour Technol 98(17):3344–3353
Ross ME, Stanley MS, Day JG, Semião AJC (2021) Removal of metals from aqueous solutions using dried Cladophora parriaudii of varying biochemical composition. J Environ Manag 290:112620
Safari GH, Zarrabi M, Hoseini M, Kamani H, Jaafari J, Mahvi AH (2015) Trends of natural and acid-engineered pumice onto phosphorus ions in aquatic environment: adsorbent preparation, characterization, and kinetic and equilibrium modeling. Desalin Water Treat 54(11):3031–3043
Salem DMSA, Moawad MN, El-Sayed AAM (2021) Comparative study for bioremediation of cobalt contaminated aqueous solutions by two types of marine macroalgae. Egypt J Aquat Res 47(1):13–19
Sheng G, Yang S, Sheng J, Zhao D, Wang X (2011) Influence of solution chemistry on the removal of Ni(II) from aqueous solution to titanate nanotubes. Chem Eng J 168(1):178–182
Shi T, Jia S, Chen Y, Wen Y, Du C, Guo H, Wang Z (2009) Adsorption of Pb(II), Cr(III), Cu(II), Cd(II) and Ni(II) onto a vanadium mine tailing from aqueous solution. J Hazard Mater 169(1):838–846
Shin WS, Kim YK (2014) Biosorption characteristics of heavy metals (Ni2+, Zn2+, Cd2+, Pb2+) from aqueous solution by Hizikia fusiformis. Environ Earth Sci 71(9):4107–4114
Sulaymon AH, Mohammed A, Al-Musawi T (2013) Column biosorption of lead, cadmium, copper, and arsenic ions onto algae. J Bioproces Biotechniq 3(1):1–7
Sweetly J, Sangeetha K, Suganthi B (2014) Biosorption of heavy metal lead from aqueous solution by nonliving biomass of Sargassum myriocystum. Int J Appl Innov Eng Manag 3:39–45
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89(2):31–59
Wei H, Binghui Z, Xia J (2016) Effect of ionic strength on phosphorus removal with modified sediments in lake: kinetics and equilibrium studies. Int J Electrochem Sci 11:9972–9986
Wu S, Xu Y, Sun J, Cao Z, Zhou J, Pan Y, Qian G (2015) Inhibiting evaporation of heavy metal by controlling its chemical speciation in MSWI fly ash. Fuel 158:764–769
Yahaya YA, Don MM (2014) Pycnoporus sanguineus as potential biosorbent for heavy metal removal from aqueous solution: a review. J Phys Sci 25(1):1
Yin J, Deng C, Yu Z, Wang X, Xu G (2018) Effective removal of lead ions from aqueous solution using nano illite/smectite clay: Isotherm, kinetic, and thermodynamic modeling of adsorption. Water 10(2):210
Acknowledgements
This work was supported by the Korea Environment Industry & Technology Institute (KEITI) through the project to develop eco-friendly new materials and processing technology derived from wildlife, funded by the Korea Ministry of Environment (MOE) (2021003240003).
Author information
Authors and Affiliations
Contributions
J.-K. Kang contributed to writing–original draft preparation, validation, data curation, and visualization; B.N. Pham contributed to methodology and formal analysis; C.-G. Lee contributed to writing–review and editing; S.-J. Park contributed to conceptualization, writing–review and editing, and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Editorial responsibility: Gaurav Sharma.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, JK., Pham, B.N., Lee, CG. et al. Biosorption of Cd2+, Cu2+, Ni2+, Pb2+ by four different macroalgae species (Costaria costata, Hizikia fusiformis, Gracilaria verrucosa, and Codium fragile). Int. J. Environ. Sci. Technol. 20, 10113–10122 (2023). https://doi.org/10.1007/s13762-022-04700-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-022-04700-z