Abstract
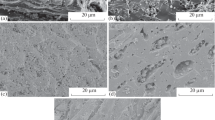
This study emphasis on the feasibility of a vegetable biosorbent Tamarix aphylla (TA) for both cationic and anionic dyes adsorption. Indeed, this plant improves its efficiency for cationic Safranin and anionic Remazol Brilliant Blue R (RBBR) dyes removal from aqueous solutions. Firstly, the physico-chemical proprieties of the stem of T. aphylla (TA) were determined by Fourier-transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), thermogravimetric analysis (TGA), and X-ray diffraction (XRD). The functional groups of T. aphylla before and after adsorption process were also determined. The parameters optimization (solution pH, biosorbent amount, biosorbent size fraction, initial dye concentration, reaction time) were taken place to control the best conditions for the adsorption process. The results showed that acidic pH (2.0) was more suitable for RBBR adsorption; however, a basic medium (pH: 8.0) was more suitable for Safranin adsorption. The pseudo-second-order model appears the suitable models for both dyes adsorption. Moreover, Temkin was the best fitting isotherm for both dyes with the highest correlation value of R2 (0.9988) and (0.9795) for Safranin and RBBR, respectively. These results explain that chemisorption of dyes takes place on the heterogeneous biomass surface. In addition, this adsorption occurred spontaneously due to the endothermic nature of the reaction. This scientific study offers a solution for colored wastewater treatment using the raw vegetable biomass T. aphylla (TA) without further chemical treatment.








Similar content being viewed by others
References
Arslantas C, Mbarek I, Saleh MA, Zelal I, Özdemir S, Dundar A, Dizge N (2022) Basic Red 18 and Remazol Brilliant Blue R biosorption using Rusulla Brevipes Agaricus Augustus, Fomes fomentarius. Water Pract Technol 17:1–3
Bensalah J, Habsaoui A, Dagdag O, Lebkiri A, Ismi I, Rifi EH, Zarrouk A (2021) Adsorption of a cationic dye (Safranin) by artificial cationic resins Amberlite®IRC-50: Equilibrium, kinetic and thermodynamic study. Chem Data Collect 35:100756
Bhatti H, Bajwa IH, Hanif MA, Bukhari I (2010) Removal of Lead and Cobalt using lignocellullosic fibers derived from Citrus reticulate waste biomass. Korean J Chem Eng 27:218–227
Dakhil IH (2020) Recycling of agriculture wastes for efficient removal of methyl orange dye using batch adsorption unit. IOP Conf Series Mater Sci Eng 881:1
Dakhil IH, Ali AH (2021) Adsorption of methylene blue dye from industrial wastewater using activated carbon prepared from agriculture wastes. Desalin Water Treat 216:372–378
Dakhil IH, Naser GF, Ali AH (2021) Assessment of modified rice husks for removal of aniline in batch adsorption process: optimization and isotherm study. J Ecol Eng 22:7
Dhahri R, Guizani M, Yilmaz M, Mechi L, Alsukaibi AKD, Alimi F, Bensalem R, Moussaoui Y (2022) Experimental design analysis of murexide dye removal by carbon produced from waste biomass material. J Chem 9:1–14
Ghosh I, Kar S, Chatterjee T, Bar N, Kumar Das S (2021) Adsorptive removal of Safranin-O dye from aqueous medium using coconut coir and its acid-treated forms: adsorption study, scale-up design, MPR and GA-ANN modeling. Sustain Chem Pharm 19:100374
Halboos AA, Sayhood MH, Hussein TA (2019) Determination celiprolol hydrochloride drug by used zero, first, second and third order derivative and peak area spectrophotometry method in its pure form and in pharmaceutical tablets. J Phys Conf Ser 1294:52035
Hameed BH (2007) Equilibrium and kinetic studies of methyl violet sorption by agricultural waste. J Hazard Mater 15:204–212
Isik Z, Saleh M, Dizge N (2021) Adsorption studies of ammonia and phosphate ions onto calcium. Surf Interfaces 26:101330
Jain M, Yadav M, Kohout T, Lahtinen M, Garg VK, Sillanpaa M (2018) Development of iron oxide/activated carbon nanoparticle composite for the removal of Cr(VI), Cu(II) and Cd(II) ions from aqueous solution. Water Resour Ind 20:54–74
Kaur S, Rani S, Mahajan RK, Asif M, Gupta VK (2015) Synthesis and adsorption properties of mesoporous material for the removal of dye Safranin: Kinetics, equilibrium, and thermodynamics. J Ind Eng Chem 22:19–27
Khani R, Sobhani S, Beyki MH (2016) Highly selective and efficient removal of lead with magnetic nano-adsorbent: multivariate optimization, isotherm and thermodynamic studies. J Colloid Interface Sci 466:198–205
Li W, Xie Z, Xue S, Ye H, Liu W, Liu Y (2021) Studies on the adsorption of dyes, Methylene blue, Safranin T, and Malachite green onto Polystyrene foam. Sep Purif Technol 276:119435
Madbouly DM, Al-Anwar A (2018) Tamarix aphylla biomass as an efficient biosorbent for removal of Pb(II) ions from aqueous solution: kinetic and applicability for different isotherm models. Egypt J Chem 61:101–119
Marco-Brown J, Guz L, Olivelli M, Schampera B, Sánchez R, Curutchet G, Candal R (2018) New insights on crystal violet dye adsorption on montmorillonite: kinetics and surface complexes studies. Chem Eng J 333:495–504
Mate ChJ, Mishra S (2020) Synthesis of borax cross-linked Jhingan gum hydrogel for remediation of Remazol Brilliant Blue R (RBBR) dye from water: adsorption isotherm, kinetic, thermodynamic and biodegradation studies. Int J Biol Macromol 151:677–690
Mbarek I, Isik Z, Ozay Y, Özdemir S, Tollu G, Moussaoui Y, Dizge N (2022) Nanocellulose synthesis from Tamarix aphylla and preparation of hybrid nanocellulose composites membranes with investigation of antioxidant and antibacterial effects. Sep Purif Technol 292:120815
Mbarek I, Slimi H, AlSukaibi AKD, Alimi F, Lajimi RH, Mechi L, Ben Salem R, Moussaoui Y (2022) Cellulose from Tamarix aphylla’s stem via acetocell for cadmium adsorption. Arab J Chem 15:103679
Mechi N, Khiari R, Elaloui E, Belgacem N (2016) Preparation of paper sheet from cellulosic fibers obtained from Prunus amygdalus and Tamarisk sp. Cellul ChemTechnol 8:863–872
Mudhoo A, Ramasamy DL, Bhatnagar A, Usman M, Sillanpaa M (2020) An analysis of the versatility and effectiveness of composts for sequestering heavy metal ions, dyes and xenobiotics from soils and aqueous milieus. Ecotoxicol Environ Saf 197:110587
Muthukkumaran A, Aravamudan K (2017) Combined Homogeneous Surface Diffusion Model – Design of experiments approach to optimize dye adsorption considering both equilibrium and kinetic aspects. J Environ Manag 204:424–435
Rahmat NA, Ali AA, Husssain SN, Muhamed MS, Kristanti RA, Hadibarata T (2016) Removal of remazol brilliant blue r from aqueous solution by adsorption using pineapple leaf powder and limon peel powder. Water Air Soil Poll 227(4):1–11
Raj A, Yadav A, Rawat AP, Singh AK, Kumar S, Pandaley AK, Sirohi R, Pandey A (2021) Kinetic and thermodynamic investigations of sewage sludge biochar in removal of Remazol Brilliant Blue R dye from aqueous solution and evaluation of residual dyes cytotoxicity. EnvironTechnol Innov 23:101556
Saad S, Davila I, Mannai F, Labidi J, Moussaoui Y (2022) Effect of autohydrolysis treatment on the integral revalorization of Ziziphus Lotus. Biomass Convers Biorefinery 1–13.
Sahu MK, Sahu UK, Patel RK (2015) Adsorption of Safranin-O dye on CO2 neutralized activated red mud waste: process modelling, analysis and optimization using statistical design. RSC Adv 5:42294
Salah M, Isik Z, Aktas Y, Arslan H, Yalvac M, Dizge N (2021) Green synthesis of zero valent iron nanoparticles using Verbascum thapsus and its Cr (VI) reduction activity. Bioresour Technol Rep 13:100637
Sayhood AA, Mohammed HJ (2015) Synthesis of new azo reagent for determination of Pd(II), Ag(I) and applied to enhance the properties of silver nano particles. Int J Chem Sci 13:1123–1136
Segal L, Creely JJ, Martin AE, Conrad CM (1959) An Empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 9:786–794
Singha J, Bhuniab H, Basua S (2018) CO2 adsorption on oxygen enriched porous carbon monoliths: Kinetics, isotherm and thermodynamic studies. J Ind Eng Chem 60:321–332
Sodhi V, Bansal A, Jha MK (2019) Minimization of Bio-sludge from tannery effluent using anoxic modified conventional activated sludge process. Environ Geotech 31:3–104
Sultanov N, Makhmoor T, Abilov ZA, Parween Z, Omurkamzinova VB, Ur-Rahman A, Choudhary MI (2001) Antioxidant and antimicrobial activities of Tamarix ramosissima. J Ethnopharmacol 78:201–205
Sun F, Xu F, Sun RC, Fowler P, Baird MS (2005) Characteristics of degraded cellulose obtained from steam-exploded wheat straw. Carbohydr Res 340:97–106
Taleb F, Ammar M, Ben Mosbah M, Ben salem R, Moussaoui Y, (2020) Chemical modification of lignin derived from spent coffee grounds for methylene blue adsorption. Sci Rep 10:11048
Tan K, Hameed B (2017) Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J Taiwan Inst Chem Eng 74:25–48
Acknowledgements
The authors express their gratitude to the Tunisian Ministry of Higher Education and Scientific Research for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have not known competing finacial interset or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Editorial responsibility: Samareh Mirkia.
Rights and permissions
About this article
Cite this article
M’barek, I., Gun, M., Moussaoui, Y. et al. Adsorption studies of cationic Safranin and anionic Remazol Brilliant Blue R dyes onto Tamarix aphylla’s stem. Int. J. Environ. Sci. Technol. 20, 4839–4850 (2023). https://doi.org/10.1007/s13762-022-04435-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-022-04435-x