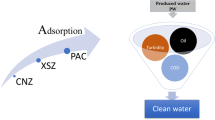
Abstract
This study investigated the removal of H2S from the effluent of the crude oil desalting plant using batch mode adsorption. Three adsorbents, including Cu2O, reboiler limescale, and activated carbon were used for H2S removal. Initial concentration, adsorbent dosage, and pH were also examined using Box–Behnken design and the H2S removal rate was considered as the response. The highest H2S removal rate for Cu2O under optimum conditions was 95%, obtained at pH = 5.6, an adsorbent dosage of 1830 mg/l, and an initial concentration of 435.9 mg/l. To achieve the highest removal of H2S, the optimum conditions were determined as pH = 9, an adsorbent dosage of 1670 mg/l, and an initial concentration of 445.7 mg/l for reboiler limescale, and pH = 5.4, an adsorbent dosage of 1750 mg/l, and an initial concentration of 282.82 mg/l for activated carbon. At these conditions, the removal percentage of H2S were 45.6 and 51.45 for reboiler limescale and activated carbon, respectively. Also for a more detailed study, the physicochemical properties of adsorbents were also investigated using FT-IR, SEM, and BET techniques.
Graphic abstract










Similar content being viewed by others
References
Aghel B, Mohadesi M, Gouran A, Razmegir MH (2020) Use of modified Iranian clinoptilolite zeolite for cadmium and lead removal from oil refinery wastewater. Int J Environ Sci Technol 17:1239–1250
Almasvandi MH, Rahimi M, Tagheie Y (2016) Microfluidic cold stripping of H2S from crude oil in low temperature and natural gas consumption. J Nat Gas Sci Eng 34:499–508
Cerny R, Rovnaníková P (2002) Transport processes in concrete. CRC Press
Gebreegziabher TB, Wang S, Nam H (2019) Adsorption of H2S, NH3 and TMA from indoor air using porous corncob activated carbon: isotherm and kinetics study. J Environ Chem Eng. 7:103234
Gialet G, Sexsmith C, Girard R, et al (2004) ATCO Midstream’s Gas Sweetening Experience Using Iron-Redox Technology, 2004, Hydrocarbon Engineering
Guo J, Luo Y, Lua AC et al (2007) Adsorption of hydrogen sulphide (H2S) by activated carbons derived from oil-palm shell. Carbon N Y 45:330–336
Habeeb OA, Kanthasamy R, Ali GAM et al (2018) Hydrogen sulfide emission sources, regulations, and removal techniques: a review. Rev Chem Eng 34:837–854
Habeeb OA, Kanthasamy R, Saber SEM, Olalere OA (2020) Characterization of agriculture wastes based activated carbon for removal of hydrogen sulfide from petroleum refinery waste water. Mater Today Proc 20:588–594
Haimour N, El-Bishtawi R, Ail-Wahbi A (2005) Equilibrium adsorption of hydrogen sulfide onto CuO and ZnO. Desalination 181:145–152
Hamed P, Mohd H, Shamsul I, Zahra ME (2014) Review of H2S sorbents at low-temperature desulfurization of biogas. Int J Chem Environ Eng 5:22–28
Hartis AE (2019) Hydrogen sulfide monitor education for use in agricultural operations
Heck HH, Hall ML, dos Santos R, Tomadakis MM (2018) Pressure swing adsorption separation of H2S/CO2/CH4 gas mixtures with molecular sieves 4A, 5A, and 13X. Sep Sci Technol 53:1490–1497
Heidaryan E (2019) A note on model selection based on the percentage of accuracy-precision. J Energy Resour Technol 141:45501
Hu Y, Watanabe M, Aida C, Horio M (2006) Capture of H2S by limestone under calcination conditions in a high-pressure fluidized-bed reactor. Chem Eng Sci 61:1854–1863
Huang Z-H, Liu G, Kang F (2012) Glucose-promoted Zn-based metal–organic framework/graphene oxide composites for hydrogen sulfide removal. ACS Appl Mater Interfaces 4:4942–4947
Lestari RAS, Sediawan WB, Syamsiah S, Teixeira JA (2016) Hydrogen sulfide removal from biogas using a salak fruit seeds packed bed reactor with sulfur oxidizing bacteria as biofilm. J Environ Chem Eng 4:2370–2377
Liu X, Wang R (2017) Effective removal of hydrogen sulfide using 4A molecular sieve zeolite synthesized from attapulgite. J Hazard Mater 326:157–164
Mohadesi M, Aghel B, Gouran A, Razmegir MH (2020) Oil refinery wastewater treatment by advanced oxidation processes for chemical oxygen demand removal using the box-behnken method. J Chem Pet Eng 54:35–46
Nguyen-Thanh D, Block K, Bandosz TJ (2005) Adsorption of hydrogen sulfide on montmorillonites modified with iron. Chemosphere 59:343–353
Ozekmekci M, Salkic G, Fellah MF (2015) Use of zeolites for the removal of H2S: a mini-review. Fuel Process Technol 139:49–60
Phooratsamee W, Hussaro K, Teekasap S, Hirunlabh J (2014) Increasing adsorption of activated carbon from palm oil shell for adsorb H2S from biogas production by impregnation. Am J Environ Sci 10:431–445
Policastro MA, Otten EJ (2007) Case files of the University of Cincinnati fellowship in medical toxicology: two patients with acute lethal occupational exposure to hydrogen sulfide. J Med Toxicol 3:73–81
Sakanishi K, Wu Z, Matsumura A et al (2005) Simultaneous removal of H2S and COS using activated carbons and their supported catalysts. Catal Today 104:94–100
Shangguan J, Zhao Y, Fan H et al (2013) Desulfurization behavior of zinc oxide based sorbent modified by the combination of Al2O3 and K2CO3. Fuel 108:80–84
Sigot L, Ducom G, Germain P (2016) Adsorption of hydrogen sulfide (H2S) on zeolite (Z): retention mechanism. Chem Eng J 287:47–53
Stepova KV, Maquarrie DJ, Krip IM (2009) Modified bentonites as adsorbents of hydrogen sulfide gases. Appl Clay Sci 42:625–628
Stirling D (2007) The sulfur problem: cleaning up industrial feedstocks. Royal Society of Chemistry
Vollertsen J, Nielsen AH, Jensen HS et al (2008) Corrosion of concrete sewers—the kinetics of hydrogen sulfide oxidation. Sci Total Environ 394:162–170
Wakker JP, Gerritsen AW, Moulijn JA (1993) High temperature H [sub 2] S and COS removal with MnO and FeO on [gamma]-Al [sub 2] O [sub 3] acceptors. Ind Eng Chem Res States) 32
Wang S, Nam H, Nam H (2020) Preparation of activated carbon from peanut shell with KOH activation and its application for H2S adsorption in confined space. J Environ Chem Eng. 8:103683
Wynnyk KG, Hojjati B, Marriott RA (2018) High-pressure sour gas and water adsorption on zeolite 13X. Ind Eng Chem Res 57:15357–15365
Yang C, Wang Y, Fan H et al (2020) Bifunctional ZnO-MgO/activated carbon adsorbents boost H2S room temperature adsorption and catalytic oxidation. Appl Catal B Environ 266:118674
Zhang H-Y, Yang C, Geng Q et al (2019) Adsorption of hydrogen sulfide by amine-functionalized metal organic framework (MOF-199): an experimental and simulation study. Appl Surf Sci 497:143815
Zhao Y, Song Z, Li X et al (2016) Metal organic frameworks for energy storage and conversion. Energy Storage Mater 2:35–62
Acknowledgements
The authors wish to thank all who assisted in conducting this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Additional information
Editorial responsibility: Lifeng Yin.
Rights and permissions
About this article
Cite this article
Karami, F., Aghel, B., Moradi, P. et al. Stripping of hydrogen sulfide from crude oil desalter effluent via different adsorbents. Int. J. Environ. Sci. Technol. 19, 5119–5130 (2022). https://doi.org/10.1007/s13762-021-03718-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-021-03718-z