Abstract
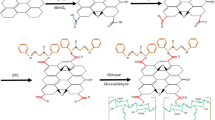
The present investigation deals with the synthesis of newly designed glucamine-functionalized magnetic graphene oxide (MGO-GLu) nanocomposite for the magnetic solid-phase extraction of inorganic arsenic (III) from water and its determination by inductively coupled plasma mass spectrometry (ICP-MS). The synthesized nanocomposite was comprehensively characterized using scanning electron microscopy (SEM), Fourier transform Infrared (FTIR) and EDX. The extraction of arsenic was optimized by analyzing various parameters, and recovery efficiency of MGO-GLu was found to be maximum at 2 mL HNO3 (0.1 M) as desorption solvent, 20 mg sorbent, 40 mL sample volume, 5 min contact time, 2 min desorption time and pH 5. Subsequently, the proposed method was validated under the optimal conditions for its figures of merit comprising limits of detection (LOD), quantification (LOQ), precision and reproducibility that were estimated to be 0.05 ng mL−1, 0.18 ng mL−1, 3.23 and 6.7% (%RSD at 10 ng mL−1, for n = 3 and n = 12 intra- and inter-day), respectively. Finally, the proposed method was applied to real water samples obtained from different sources (river, tap and industrial wastewater) displaying a high recovery range of 83–104%. Thus, the designed nanocomposite sorbent offers ease of applicability, rapid analysis, ease of isolation and efficiency for the trace determination of inorganic arsenic in water environmental samples.










Similar content being viewed by others
References
Abbaszadeh S, Nodeh HR, Alwi SRW (2018) Bio-adsorbent derived from papaya peel waste and magnetic nanoparticles fabricated for lead determination. Pure Appl Chem 90:79–92
Ahmad NF, Kamboh MA, Nodeh HR, Halim SNBA, Mohamad S (2017) Synthesis of piperazine functionalized magnetic sporopollenin: a new organic-inorganic hybrid material for the removal of lead(II) and arsenic(III) from aqueous solution. Environ Sci Pollut Res 24:21846–21858. https://doi.org/10.1007/s11356-017-9820-9
Ali J, Tuzen M, Kazi TG, Hazer B (2016) Inorganic arsenic speciation in water samples by miniaturized solid phase microextraction using a new polystyrene polydimethyl siloxane polymer in micropipette tip of syringe system. Talanta 161:450–458
Baker JA, Ayad FK, Maitham SA (2016) Influence of various parameters on the levels of arsenic in washed scalp hair from Karbala, Iraq by using ICP-OES technique. Karbala Int J Mod Sci 2:104–112
Chen L, Wang T, Tong J (2011) Application of derivatized magnetic materials to the separation and the preconcentration of pollutants in water samples. TrAC Trends Anal Chem 30:1095–1108
de Melo Pinheiro AF, Nijmeijer A, Sripathi VGP, Winnubst L (2015) Chemical modification/grafting of mesoporous alumina with polydimethylsiloxane (PDMS). Eur J Chem 6:287–295
de Namor AFD, Al Hakawati N, Hamdan WA, Soualhi R, Korfali S, Valiente L (2017) Calix [4] pyrrole for the removal of arsenic (III) and arsenic (V) from water. J Hazard Mater 326:61–68
Dehghani MH, Tajik S, Panahi A, Khezri M, Zarei A, Heidarinejad Z, Yousefi M (2018) Adsorptive removal of noxious cadmium from aqueous solutions using poly urea-formaldehyde: a novel polymer adsorbent. MethodsX 5:1148–1155
EU (1998) European Community, Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Off J Eur Communities L330:32–54
Feng Q, Zhang Z, Ma Y, He X, Zhao Y, Chai Z (2012) Adsorption and desorption characteristics of arsenic onto ceria nanoparticles. Nanoscale Res Lett 7:84
Gabris MA, Jume BH, Rezaali M, Shahabuddin S, Rashidi Nodeh H, Saidur R (2018) Novel magnetic graphene oxide functionalized cyanopropyl nanocomposite as an adsorbent for the removal of Pb(II) ions from aqueous media: equilibrium and kinetic studies. Environ Sci Pollut Res 25:27122–27132. https://doi.org/10.1007/s11356-018-2749-9
Gao J, Feng K, Li H, Jiang Y, Zhou L (2015) Immobilized lipase on porous ceramic monoliths for the production of sugar-derived oil gelling agent. RSC Adv 5:68601–68609
Huang C, Xie W, Li X, Zhang J (2011) Speciation of inorganic arsenic in environmental waters using magnetic solid phase extraction and preconcentration followed by ICP-MS. Microchim Acta 173:165–172
Kamboh MA, Yilmaz M (2013) Synthesis of N-methylglucamine functionalized calix [4] arene based magnetic sporopollenin for the removal of boron from aqueous environment. Desalination 310:67–74
Kamboh MA, Wan Ibrahim WA, Rashidi Nodeh H, Sanagi MM, Sherazi STH (2016) The removal of organophosphorus pesticides from water using a new amino-substituted calixarene-based magnetic sporopollenin. New J Chem 40:3130–3138. https://doi.org/10.1039/C5NJ02284C
Khan NA, Khan SU, Ahmed S, Farooqi IH, Yousefi M, Mohammadi AA, Changani F (2020) Recent trends in disposal and treatment technologies of emerging-pollutants-A critical review. TrAC Trends Anal Chem 122:115744
Kumar S, Terashima C, Fujishima A, Krishnan V, Pitchaimuthu S (2019) Photocatalytic degradation of organic pollutants in water using graphene oxide composite. In: A new generation material graphene: applications in water technology, Springer, pp 413–438
Kumari P, Sharma P, Srivastava S, Srivastava MM (2006) Biosorption studies on shelled Moringa oleifera Lamarck seed powder: removal and recovery of arsenic from aqueous system. Int J Miner Process 78:131–139
Lai GS, Lau WJ, Goh PS, Ismail AF, Yusof N, Tan YH (2016) Graphene oxide incorporated thin film nanocomposite nanofiltration membrane for enhanced salt removal performance. Desalination 387:14–24
Liang P, Liu Y, Guo L, Zeng J, Lu H (2004) Multiwalled carbon nanotubes as solid-phase extraction adsorbent for the preconcentration of trace metal ions and their determination by inductively coupled plasma atomic emission spectrometry. J Anal At Spectrom 19:1489–1492
Lin Z, Puls RW (2000) Adsorption, desorption and oxidation of arsenic affected by clay minerals and aging process. Environ Geol 39:753–759
Liu F, De Cristofaro A, Violante A (2001) Effect of pH, phosphate and oxalate on the adsorption/desorption of arsenate on/from goethite. Soil Sci 166:197–208
Magoda C, Nomngongo PN, Mabuba N (2016) Magnetic iron–cobalt/silica nanocomposite as adsorbent in micro solid-phase extraction for preconcentration of arsenic in environmental samples. Microchem J 128:242–247
Mihucz VG, Enesei D, Veszely Á, Bencs L, Pap-Balázs T, Óvári M, Streli C, Záray G (2017) A simple method for monitoring of removal of arsenic species from drinking water applying on-site separation with solid phase extraction and detection by atomic absorption and X-ray fluorescence based techniques. Microchem J 135:105–113
Mohammadi Nodeh MK, Gabris MA, Rashidi Nodeh H, Esmaeili Bidhendi M (2018a) Efficient removal of arsenic(III) from aqueous media using magnetic polyaniline-doped strontium–titanium nanocomposite. Environ Sci Pollut Res 25:16864–16874. https://doi.org/10.1007/s11356-018-1870-0
Mohammadi Nodeh MK, Soltani S, Shahabuddin S, Rashidi Nodeh H, Sereshti H (2018b) Equilibrium, kinetic and thermodynamic study of magnetic polyaniline/graphene oxide based nanocomposites for ciprofloxacin removal from water. J Inorg Organomet Polym Mater 28:1226–1234. https://doi.org/10.1007/s10904-018-0782-2
Mousavi SV, Bozorgian A, Mokhtari N, Gabris MA, Nodeh HR, Ibrahim WAW (2019) A novel cyanopropylsilane-functionalized titanium oxide magnetic nanoparticle for the adsorption of nickel and lead ions from industrial wastewater: equilibrium, kinetic and thermodynamic studies. Microchem J 145:914–920
Murugesan GS, Sathishkumar M, Swaminathan K (2006) Arsenic removal from groundwater by pretreated waste tea fungal biomass. Bioresour Technol 97:483–487
Musa M, Wan Ibrahim WA, Mohd Marsin F, Abdul Keyon AS, Rashidi Nodeh H (2018) Graphene-magnetite as adsorbent for magnetic solid phase extraction of 4-hydroxybenzoic acid and 3,4-dihydroxybenzoic acid in stingless bee honey. Food Chem. https://doi.org/10.1016/j.foodchem.2018.04.020
Ngeontae W, Aeungmaitrepirom W, Tuntulani T (2007) Chemically modified silica gel with aminothioamidoanthraquinone for solid phase extraction and preconcentration of Pb(II), Cu (II), Ni (II), Co (II) and Cd (II). Talanta 71:1075–1082
Pompeo F, Resasco DE (2002) Water solubilization of single-walled carbon nanotubes by functionalization with glucosamine. Nano Lett 2:369–373
Pourghazi K, Amoli-Diva M, Beiraghi A (2015) Speciation of ultra-trace amounts of inorganic arsenic in water and rice samples by electrothermal atomic absorption spectrometry after solid-phase extraction with modified Fe3O4 nanoparticles. Int J Environ Anal Chem 95:324–338
Radfard M, Yunesian M, Nabizadeh R, Biglari H, Nazmara S, Hadi M, Yousefi N, Yousefi M, Abbasnia A, Mahvi AH (2018) Drinking water quality and arsenic health risk assessment in Sistan and Baluchestan, Southeastern Province, Iran. Hum Ecol Risk Assess An Int J 25:949–965
Rashidi Nodeh H, Wan Ibrahim WA, Ali I, Sanagi MM (2016a) Development of magnetic graphene oxide adsorbent for the removal and preconcentration of As(III) and As(V) species from environmental water samples. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-016-6137-z
Rashidi Nodeh H, Wan Ibrahim WA, Ali I, Sanagi MM (2016b) Development of magnetic graphene oxide adsorbent for the removal and preconcentration of As (III) and As (V) species from environmental water samples. Environ Sci Pollut Res 23:9759–9773. https://doi.org/10.1007/s11356-016-6137-z
Roy E, Patra S, Madhuri R, Sharma PK (2016) Europium doped magnetic graphene oxide-MWCNT nanohybrid for estimation and removal of arsenate and arsenite from real water samples. Chem Eng J 299:244–254
Saleh HN, Panahande M, Yousefi M, Asghari FB, Conti GO, Talaee E, Mohammadi AA (2019) Carcinogenic and non-carcinogenic risk assessment of heavy metals in groundwater wells in Neyshabur Plain. Iran. Biol Trace Elem Res 190:251–261
Sayin S, Ozcan F, Yilmaz M (2010) Synthesis and evaluation of chromate and arsenate anions extraction ability of a N-methylglucamine derivative of calix [4] arene immobilized onto magnetic nanoparticles. J Hazard Mater 178:312–319
Shamsollahi HR, Alimohammadi M, Momeni S, Naddafi K, Nabizadeh R, Khorasgani FC, Masinaei M, Yousefi M (2019) Assessment of the health risk induced by accumulated heavy metals from anaerobic digestion of biological sludge of the lettuce. Biol Trace Elem Res 188:514–520
Sharifi S, Nabizadeh R, Akbarpour B, Azari A, Ghaffari HR, Nazmara S, Mahmoudi B, Shiri L, Yousefi M (2019) Modeling and optimizing parameters affecting hexavalent chromium adsorption from aqueous solutions using Ti-XAD7 nanocomposite: RSM-CCD approach, kinetic, and isotherm studies. J Environ Heal Sci Eng 17:873–888
Sigdel A, Park J, Kwak H, Park P-K (2016) Arsenic removal from aqueous solutions by adsorption onto hydrous iron oxide-impregnated alginate beads. J Ind Eng Chem 35:277–286
Sivrikaya S, Imamoglu M (2018) Online solid-phase extraction of Cd (II), Cu (II), and Co (II) using covalently attached bis (salicylaldimine) to silica gel for determination in food and water by flame atomic absorption spectrometry. Anal Lett 51:773–791
Srivastava NK, Majumder CB (2008) Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J Hazard Mater 151:1–8
Van Herreweghe S, Swennen R, Vandecasteele C, Cappuyns V (2003) Solid phase speciation of arsenic by sequential extraction in standard reference materials and industrially contaminated soil samples. Environ Pollut 122:323–342
Wan Ibrahim WA, Nodeh HR, Aboul-Enein HY, Sanagi MM (2015) Magnetic solid-phase extraction based on modified ferum oxides for enrichment, preconcentration, and isolation of pesticides and selected pollutants. Crit Rev Anal Chem 45:270–287. https://doi.org/10.1080/10408347.2014.938148
Wan Ibrahim WA, Rashidi Nodeh H, Sanagi MM (2016) Graphene-based materials as solid phase extraction sorbent for trace metal ions, organic compounds, and biological sample preparation. Crit Rev Anal Chem 46:267–283. https://doi.org/10.1080/10408347.2015.1034354
Wang C, Feng C, Gao Y, Ma X, Wu Q, Wang Z (2011) Preparation of a graphene-based magnetic nanocomposite for the removal of an organic dye from aqueous solution. Chem Eng J 173:92–97
Wang N, Wei RQ, Cao FT, Liu XN (2012) Synthesis of a new acetyl-meglumine resin and its adsorption properties of boron. Chem J Chinese Univ 12:36
World Health Organisation (WHO) Guidelines for Drinking-Water Quality, 4rt Ed., (Geneva, Switzerland, 2011)
Zare EN, Motahari A, Sillanpää M (2018) Nanoadsorbents based on conducting polymer nanocomposites with main focus on polyaniline and its derivatives for removal of heavy metal ions/dyes: a review. Environ Res 162:173–195
Zhang J, Zhang Q, Vo TT, Parrish DA, Shreeve JM (2015) Energetic salts with π-stacking and hydrogen-bonding interactions lead the way to future energetic materials. J Am Chem Soc 137:1697–1704
Zhou Q, Zheng Z, Xiao J, Fan H (2016) Sensitive determination of As (III) and As (V) by magnetic solid phase extraction with Fe@ polyethyleneimine in combination with hydride generation atomic fluorescence spectrometry. Talanta 156:196–203
Acknowledgements
The authors would like to thank Department of Science, University of Tehran, Standard Research Institute, School of Technology, Pandit Deendayal Petroleum University, Gandhinagar, Gujarat, and Tan university of Vietnam for the research facilities and financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Hari Pant.
Rights and permissions
About this article
Cite this article
Gabris, M.A., Hadi Jume, B., Sadegh Amiri, I. et al. Magnetic graphene oxide nanocomposite functionalized with glucamine for the trace extraction of arsenic (III) from aqueous media. Int. J. Environ. Sci. Technol. 18, 1109–1118 (2021). https://doi.org/10.1007/s13762-020-02854-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-020-02854-2