Abstract
Nanosilica has a number of properties that pose advantages in a range of applications. In this study, we performed a series of experiments to prepare nanosilica particles from blast furnace slag. The collected samples were initially treated using nitric acid to remove insoluble silica, and the influence of various key factors on the silica extraction efficiency was examined. The optimum values of liquid-to-solid ratio, temperature, leaching time, and acid concentration were 2.3:1, 120 °C, 90 min, and 50%, respectively. Optimal silica nanoparticles were obtained via filtration and modification using cetyltrimethyl ammonium bromide, a cationic surfactant. The specific surface area (SBET) of unmodified silica was 303.83 m2/g, while that of silica particles modified with cetyltrimethyl ammonium bromide increased to 1506 m2/g, corresponding to ultrafine particles. In addition, this research was performed using a waste by-product and then converted it into a high-quality, valuable product, which can help to mitigate the significant environmental problems caused by blast furnace slag waste in both China and Pakistan. This study may be used as a reference for nanosilica particle preparation from blast furnace slag.










Similar content being viewed by others
Change history
17 September 2019
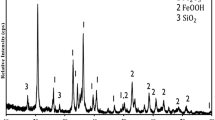
The original version of this article unfortunately contained a mistake in Figure 1.
References
Ahmed ZT, Hand DW, Watkins MK, Sutter LL (2014) Combined adsorption isotherms for measuring the adsorption capacity of fly ash in concrete. ACS Sustain Chem Eng 2(4):614–620
Arai Y, Powell BA, Kaplan DI (2017) Sulfur speciation in untreated and alkali treated ground-granulated blast furnace slag. Sci Total Environ 589:117–121
Bai G, Teng W, Wang X, Zhang H, Xu P (2010) Processing and kinetics studies on the alumina enrichment of coal fly ash by fractionating silicon dioxide as nano particles. Fuel Process Technol 91:175–184
Bai J, Li Y, Ren L, Mao M, Zeng M, Zhao X (2015) Thermal insulation monolith of aluminum tobermorite nanosheets prepared from fly ash. ACS Sustain Chem Eng 3:2866–2873
Bandura L, Franus M, Józefaciuk G, Franus W (2015) Synthetic zeolites from fly ash as effective mineral sorbents for land-based petroleum spills cleanup. Fuel 147:100–107
Deng M, Zhang G, Zeng Y, Pei X, Huang R, Lin J (2016) Simple process for synthesis of layered sodium silicates using rice husk ash as silica source. J Alloys Compd 683:412–417
Du C, Yang H (2012) Investigation of the physicochemical aspects from natural kaolin to Al-MCM-41 mesoporous materials. J Colloid Interface Sci 369:216–222
Fu P, Yang T, Feng J, Yang H (2015) Synthesis of mesoporous silica MCM-41 using sodium silicate derived from copper ore tailings with an alkaline molted-salt method. Ind Eng Chem 29:338–343
Gerardin C, Reboul J, Bonne M, Lebeau B (2013) Ecodesign of ordered mesoporous silica materials. Chem Soc Rev 42:4217–4255
Ghorbani F, Habibollah Y, Mehraban Z, Çelik MS, Ghoreyshi AA, Anbia M (2013) Preparation and characterization of highly pure silica from sedge as agricultural waste and its utilization in the synthesis of mesoporous silica MCM-41. J Taiwan Inst Chem E 44:821–828
Giri SK, Das NN, Pradhan GC (2011a) Magnetite powder and kaolinite derived from waste iron ore tailings for environmental applications. Powder Technol 214:513–518
Giri SK, Das NN, Pradhan GC (2011b) Synthesis and characterization of magnetite nanoparticles using waste iron ore tailings for adsorptive removal of dyes from aqueous solution. C Colloids Surf A Physicochem Eng Asp 389:43–49
Guo X, Ding L, Kanamori K, Nakanishi K, Yang H (2017) Functionalization of hierarchically porous silica monoliths with polyethyleneimine (PEI) for CO2 adsorption. Microporous Mesoporous Mater 245:51–57
Halina M, Ramesh S, Yarmo MA, Kamarudin RA (2007) Non-hydrothermal synthesis of mesoporous materials using sodium silicate from coal fly ash. Mater Chem Phys 101:344–351
Hui KS, Chao CY (2006) Synthesis of MCM-41 from coal fly ash by a green approach: influence of synthesis pH. J Hazard Mater 137:1135–1148
Hui KS, Chao CY, Kot SC (2005) Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash. J Hazard Mater 127:89–101
Jang HT, Park Y, Ko YS, Lee JY, Margandan B (2009) Highly siliceous MCM-48 from rice husk ash for CO2 adsorption. Int J Greenh Gas Con 3:545–549
Kim S-J, Seo S-G, Jung S-C (2010) Preparation of high purity nano silica particles from blast-furnace slag. Korean J Chem Eng 27(6):1901–1905
Kutchko B, Kim A (2006) Fly ash characterization by SEM–EDS. Fuel 85:2537–2544
Li D, Min H, Jiang X, Ran X, Zou L, Fan J (2013) One-pot synthesis of Aluminum-containing ordered mesoporous silica MCM-41 using coal fly ash for phosphate adsorption. J Colloid Interface Sci 404:42–48
Li KM, Jiang JG, Tian SC, Chen XJ, Yan F (2014) Influence of silica types on synthesis and performance of amine-silica hybrid materials used for CO2 capture. J Phys Chem 118:2454–2462
Liang YL, Pearson RA (2010) The toughening mechanism in hybrid epoxy-silica-rubber nanocomposites (HESRNs). Polymer 51(21):4880–4890
Lin LY, Kuo JT, Bai H (2011) Silica materials recovered from photonic industrial waste powder: its extraction, modification, characterization and application. J Hazard Mater 192:255–262
Liou TH (2011) A green route to preparation of MCM-41 silicas with well-ordered mesostructure controlled in acidic and alkaline environments. Chem Eng J 171:1458–1468
Majchrzak KI (2011) Thermogravimetry applied to characterization of fly ash-based MCM-41 mesoporous materials. J Therm Anal Calorim 107:911–921
Mohammadi F, Mohammadi T (2017) Optimal conditions of porous ceramic membrane synthesis based on alkali activated blast furnace slag using Taguchi method. Ceram Int 43:14369–14379
Moore JK, Sakwa-Novak MA, Chaikittisilp W, Mehta AK, Conradi MS, Jones CW, Hayes SE (2015) Characterization of a mixture of CO2 adsorption products in hyperbranched aminosilica adsorbents by (13)C solid-state NMR. Environ Sci Technol 49:13684–13691
Saha S, Rajasekaran C (2017) Enhancement of the properties of fly ash based geopolymer paste by incorporating ground granulated blast furnace slag. Constr Build Mater 146:615–620
Slowing II, Trewyn BG, Giri S, Lin VSY (2007) Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv Funct Mater 17:1225–1236
Teng W (2013) Rapid and efficient removal of microcystins by ordered mesoporous silica. Environ Sci Technol 47:8633–8641
Valverde JM, Miranda-Pizarro J, Perejón A, Sánchez-Jiménez PE, Pérez-Maqueda LA (2017) Calcium-looping performance of steel and blast furnace slags for thermochemical energy storage in concentrated solar power plants. J CO2 Util 22:143–154
Wang W, Martin JC, Fan X, Han A, Luo Z, Sun L (2012) Silica nanoparticles and frameworks from rice husk biomass. ACS Appl Mater Interfaces 4:977–981
Witoon T (2012) Polyethyleneimine-loaded bimodal porous silica as low-cost and high-capacity sorbent for CO2 capture. Mater Chem Phys 137:235–245
Xie Y, Zhang Y, Ouyang J, Yang H (2014) Mesoporous material Al-MCM-41 from natural halloysite. Phys Chem Miner 41:497–503
Yan F, Jiang J, Chen X, Tian S, Li K (2014) Synthesis and characterization of silica nanoparticles preparing by low-temperature vapor-phase hydrolysis of SiCl4. Ind Eng Chem Res 53:11884–11890
Yan F, Jiang J, Li K, Tian S, Zhao M, Chen X (2015) Performance of coal fly ash stabilized, CaO-based sorbents under different carbonation-calcination conditions. ACS Sustain Chem Eng 3:2092–2099
Yang G, Deng Y, Wang J (2014) Non-hydrothermal synthesis and characterization of MCM-41 mesoporous materials from iron ore tailing. Ceram Int 40:7401–7406
Yu Z, Wang Y, Liu X, Sun J, Sha G, Yang J, Meng C (2014) A novel pathway for the synthesis of ordered mesoporous silica from diatomite. Mater Lett 119:150–153
Zeng W, Bai H (2014) Swelling-agent-free synthesis of rice husk derived silica materials with large mesopores for efficient CO2 capture. Chem Eng J 251:1–9
Zhao M, Yang X, Church TL, Harris AT (2012) Novel CaO–SiO2 sorbent and bifunctional Ni/Co–CaO/SiO2 complex for selective H2 synthesis from cellulose. Environ Sci Technol 46:2976–2983
Zhao Y, Gong J, Zhao S (2017) Experimental study on shrinkage of HPC containing fly ash and ground granulated blast-furnace slag. Constr Build Mater 155:145–153
Zhou C, Gao Q, Luo W, Zhou Q, Wang H, Yan C, Duan P (2015) Preparation, characterization and adsorption evaluation of spherical mesoporous Al-MCM-41 from coal fly ash. J Taiwan Inst Chem E 52:147–157
Acknowledgements
We are thankful for the kind and timely cooperation of Beijing National Center for Electron Microscopy (BNEM) at Tsinghua University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Fatih ŞEN.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fakhar, H., Jiang, J. A zero-waste approach to blast furnace slag by synthesis of mesoporous nanosilica with high surface area. Int. J. Environ. Sci. Technol. 17, 309–318 (2020). https://doi.org/10.1007/s13762-019-02492-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-019-02492-3