Abstract
Magnesium–aluminum-layered double hydroxides (LDH) and proteins extracted from bael or bilva oil meal (BP) were used to form composites (LDH-BP) by a simple co-precipitation method. The synthesized composites were utilized to remove lead ions from contaminated water and to study the antimicrobial and antioxidant activities. BPs have inherent antimicrobial and anticancer properties and are biodegradable. Combining BP with LDH will result in unique composites having high affinity for sorption and also antibacterial properties, ideal for multipurpose water purification applications. The LDH-BP showed high affinity for Pb(II) ions with removal efficiency as high as 90% from a solution having an initial lead concentration of 100 mg/l. Maximum adsorption was noted at room temperature at a pH of 5. The level of sorption achieved was considerably high for Pb(II) compared to other biomaterials used earlier. Sorption was found to follow Langmuir and Freundlich isotherms and pseudo-second-order kinetics with R2 values greater than 0.99. The LDH-BP composite showed maximum adsorption capacity of 625.00 mg g−1. Additionally, it exhibited good antioxidant activity by inhibiting 42–50% of DPPH free radicals in the DPPH concentration range of 10–50 µl. The composite also eliminated the Streptococcus coliform bacteria completely upon 6 h of incubation. The filtrate obtained after the removal of lead ions using the LDH-BP composite could retain its antibacterial activity up to 14 h. The low-cost multifunctional materials developed in this research could lead to new technologies and products for water purification.
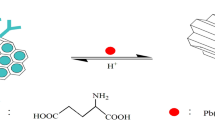
Graphical abstract
Multi-functional LDH–protein composite has been synthesized by a simple co-precipitation method and used for the removal of heavy metal ions. Also antioxidant and antibacterial activity of the composite have been investigated.








Similar content being viewed by others
References
Ahmad T, Rafatullah M, Ghazali A, Sulaiman O, Hashim R (2011) Oil palm biomass-based adsorbents for the removal of water pollutants: a review. J Environ Sci Health Part C 29:177–222
Ahmad T, Danish M, Rafatullah M, Ghazali A, Sulaiman O, Hashim R, Ibrahim MNM (2012) The use of date palm as a potential adsorbent for wastewater treatment: a review. Environ Sci Pollut Res 19:1464–1484
Anandkumar J, Mandal B (2009) Removal of Cr(VI) from aqueous solution using Bael fruit (Aegle marmelos correa) shell as an adsorbent. J Hazard Mater 168:633–640
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200
Cavani F, Trifirò F, Vaccari A (1991) Hydrotalcite-type anionic clays: preparation, properties and applications. Catal Today 11:173–301
Chakravarty S et al (2010) Removal of Pb(II) ions from aqueous solution by adsorption using bael leaves (Aegle marmelos). J Hazard Mater 173:502–509
Elkady M, Mahmoud M, Abd-El-Rahman H (2011) Kinetic approach for cadmium sorption using microwave synthesized nano-hydroxyapatite. J Non-Cryst Solids 357:1118–1129
Goh K-H, Lim T-T, Dong Z (2008) Application of layered double hydroxides for removal of oxyanions: a review. Water Res 42:1343–1368
Gong J, Liu T, Wang X, Hu X, Zhang L (2011) Efficient removal of heavy metal ions from aqueous systems with the assembly of anisotropic layered double hydroxide nanocrystals@ carbon nanosphere. Environ Sci Technol 45:6181–6187
Guo Y, Zhu Z, Qiu Y, Zhao J (2013) Synthesis of mesoporous Cu/Mg/Fe layered double hydroxide and its adsorption performance for arsenate in aqueous solutions. J Environ Sci 25:944–953
Herney-Ramirez J, Vicente MA, Madeira LM (2010) Heterogeneous photo-fenton oxidation with pillared clay-based catalysts for wastewater treatment: a review. Appl Catal B 98:10–26
Hutson ND, Speakman SA, Payzant EA (2004) Structural effects on the high temperature adsorption of CO2 on a synthetic hydrotalcite. Chem Mater 16:4135–4143
Ibrahim MM, Ngah WW, Norliyana M, Daud WW, Rafatullah M, Sulaiman O, Hashim R (2010) A novel agricultural waste adsorbent for the removal of lead(II) ions from aqueous solutions. J Hazard Mater 182:377–385
Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182
Jerri HA, Adolfsen KJ, McCullough LR, Velegol D, Velegol SB (2011) Antimicrobial sand via adsorption of cationic Moringa oleifera protein. Langmuir 28:2262–2268
Li J, Fan Q, Wu Y, Wang X, Chen C, Tang Z, Wang X (2016) Magnetic polydopamine decorated with Mg–Al LDH nanoflakes as a novel bio-based adsorbent for simultaneous removal of potentially toxic metals and anionic dyes. J Mater Chem A 4:1737–1746
Liang X, Hou W, Xu J (2009) Sorption of Pb(II) on Mg–Fe layered double hydroxide. Chin J Chem 27:1981–1988
Liang X, Hou W, Xu Y, Sun G, Wang L, Sun Y, Qin X (2010) Sorption of lead ion by layered double hydroxide intercalated with diethylenetriaminepentaacetic acid. Colloids Surf A 366:50–57
Lv L, He J, Wei M, Evans D, Duan X (2006) Factors influencing the removal of fluoride from aqueous solution by calcined Mg–Al–CO3 layered double hydroxides. J Hazard Mater 133:119–128
Ma L, Wang Q, Islam SM, Liu Y, Ma S, Kanatzidis MG (2016) Highly selective and efficient removal of heavy metals by layered double hydroxide intercalated with the MoS4 2− ion. J Am Chem Soc 138:2858–2866
Newman SP, Jones W (1998) Synthesis, characterization and applications of layered double hydroxides containing organic guests. New J Chem 22:105–115
Olivera S, Muralidhara HB, Venkatesh K, Guna VK, Gopalakrishna K, Kumar Y (2016) Potential applications of cellulose and chitosan nanoparticles/composites in wastewater treatment: a review. Carbohyd Polym 153:600–618
Reddy MR, Xu Z, Lu G, Da Costa JD (2008) Influence of water on high-temperature CO2 capture using layered double hydroxide derivatives. Ind Eng Chem Res 47:2630–2635
Rusin PA, Rose JB, Haas CN, Gerba CP (1997) Risk assessment of opportunistic bacterial pathogens in drinking water. Rev Environ Contam Toxicol 152:57–83
Shan R-R, Yan L-G, Yang K, Hao Y-F, Du B (2015) Adsorption of Cd(II) by Mg–Al–CO3-and magnetic Fe3O4/Mg–Al–CO3-layered double hydroxides: Kinetic, isothermal, thermodynamic and mechanistic studies. J Hazard Mater 299:42–49
Singh S, Barick K, Bahadur D (2013) Fe3O4 embedded ZnO nanocomposites for the removal of toxic metal ions, organic dyes and bacterial pathogens. J Mater Chem A 1:3325–3333
Sun X, Yang L, Li Q, Zhao J, Li X, Wang X, Liu H (2014) Amino-functionalized magnetic cellulose nanocomposite as adsorbent for removal of Cr(VI): synthesis and adsorption studies. Chem Eng J 241:175–183
Tahir S, Rauf N (2006) Removal of a cationic dye from aqueous solutions by adsorption onto bentonite clay. Chemosphere 63:1842–1848
Upadhyay RK, Soin N, Roy SS (2014) Role of graphene/metal oxide composites as photocatalysts, adsorbents and disinfectants in water treatment: a review RSC. Advances 4:3823–3851
Vakili M, Rafatullah M, Ibrahim MH, Abdullah AZ, Salamatinia B, Gholami Z (2014) Oil palm biomass as an adsorbent for heavy metals. Rev Environ Contam Toxicol 232:61–88
Visa M, Chelaru A-M (2014) Hydrothermally modified fly ash for heavy metals and dyes removal in advanced wastewater treatment. Appl Surf Sci 303:14–22
Wilson O et al (1999) Surface and interfacial properties of polymer-intercalated layered double hydroxide nanocomposites. Appl Clay Sci 15:265–279
Xu P et al (2012) Use of iron oxide nanomaterials in wastewater treatment: a review. Sci Total Environ 424:1–10
Zhang M, Gao B, Yao Y, Inyang M (2013a) Phosphate removal ability of biochar/MgAl-LDH ultra-fine composites prepared by liquid-phase deposition. Chemosphere 92:1042–1047
Zhang W, Shi X, Zhang Y, Gu W, Li B, Xian Y (2013b) Synthesis of water-soluble magnetic graphene nanocomposites for recyclable removal of heavy metal ions. J Mater Chem A 1:1745–1753
Zhang F, Song Y, Song S, Zhang R, Hou W (2015) Synthesis of magnetite–graphene oxide-layered double hydroxide composites and applications for the removal of Pb(II) and 2,4-dichlorophenoxyacetic acid from aqueous solutions. ACS Appl Mater Interfaces 7:7251–7263
Zhao D, Sheng G, Hu J, Chen C, Wang X (2011) The adsorption of Pb(II) on Mg2Al layered double hydroxide. Chem Eng J 171:167–174
Zhu C-S, Wang L-P, W-b C (2009) Removal of Cu (II) from aqueous solution by agricultural by-product: peanut hull. J Hazard Mater 168:739–746
Zhuang Y, Yu F, Ma J, Chen J (2015) Adsorption of ciprofloxacin onto graphene–soy protein biocomposites. New J Chem 39:3333–3336
Zhuang Y, Yu F, Ma J, Chen J (2016) Facile synthesis of three-dimensional graphene–soy protein aerogel composites for tetracycline adsorption. Desalin Water Treat 57:9510–9519
Acknowledgements
The authors thank and acknowledge Dr. Yogesh Kumar, Associate Professor, Jain University and Ms. Archana, Assistant Professor, Jain University, for their assistance.
Authors contribution
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Editorial responsibility: M. Abbaspour.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Olivera, S., Hu, C., Nagananda, G.S. et al. Multipurpose composite for heavy metal sorption, antimicrobial, and antioxidant applications. Int. J. Environ. Sci. Technol. 16, 2017–2030 (2019). https://doi.org/10.1007/s13762-018-1774-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-018-1774-z