Abstract
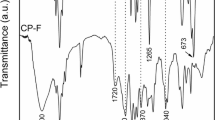
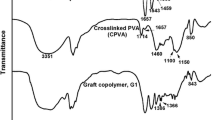
The research includes the synthesis of copolymeric material and its chemical modification in order to enhance its adsorptive properties toward copper ions removal. The copolymeric material of acrylonitrile (AN) and styrene (St) was prepared in the ratio of 70:30% V/V, respectively, using 1-propanol as a co-solvent using the precipitation polymerization technique. The prepared copolymeric material undergoes an alkaline modification process using sodium hydroxide. The influence of the modification process variables has been studied on the removal of copper ions where the agitation rate was found to be the most effective parameter in this process. The copolymer chemical structure was investigated through Fourier-transform infrared spectrum, indicating the evaluation of carboxylate groups at 3497 cm−1 after sodium hydroxide treatment. The copolymer thermal stability has been increased by the modification process which was confirmed by thermal characterization. The morphological structure and particle size of the copolymer particles were investigated to be 53.6 nm. The removal process parameters have been studied to show pH variation as a critical parameter in this stage of application. The maximum removal percent was achieved after 90 min to reach 89.6%. The adsorption capacity of the modified (St–AN) copolymer to copper solution was found to be 20 g/L. Thermodynamic, isothermal, and kinetic studies were done where it showed the adsorption process to be spontaneous, thermodynamically favorable, and endothermic in nature.









Similar content being viewed by others
References
Abo-El-Enein S, Eissa M, Diafullah A, Rizk M, Mohamed F (2009) Removal of some heavy metals ions from wastewater by copolymer of iron and aluminum impregnated with active silica derived from rice husk ash. J Hazard Mater 172:574–579
Ajmal M, Khan Rao RA, Ahmad R (2011) Adsorption studies of heavy metals on Tectona grandis: removal and recovery of Zn (II) from electroplating wastes. J Dispers Sci Technol 32:851–856
Akpor OB, Ohiobor G, Olaolu T (2014) Heavy metal pollutants in wastewater effluents: sources, effects and remediation. Adv Biosci Bioeng 2:37–43
Al-Degs Y, Khraisheh MAM, Allen SJ, Ahmad MN (2000) Effect of carbon surface chemistry on the removal of reactive dyes from textile effluent. Water Res 34:927–935
Anwar J, Shafique U, Salman M, Dar A, Anwar S (2010) Removal of Pb(II) and Cd (II) from water by adsorption on peels of banana. Biores Technol 101:1752–1755
Babarinde NAA, Oyebamiji J, Sanni RA (2006) Biosorption of lead ions from aqueous solution by maize leaf. Int J Phys Sci 1:23–26
Babel S, Kurniawan TA (2004) Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere 54:951–967
Chang Q, Wang G (2007) Study on the macromolecular coagulant PEX which traps heavy metals. Chem Eng Sci 62:4636–4643
Chiou M-S, Li H-Y (2002) Equilibrium and kinetic modeling of adsorption of reactive dye on cross-linked chitosan beads. J Hazard Mater 93:233–248
El-Aassar MR, Masoud MS, Elkady MF, Elzain AA (2017) Synthesis, optimization, and characterization of poly(Styrene-co-Acrylonitrile) copolymer prepared via precipitation polymerization. Adv Polym Technol 37(6):2021–2029
El-Aassar M, Fakhry H, Elzain AA, Farouk H, Hafez EE (2018) Rhizofiltration system consists of chitosan and natural Arundo donax L. for removal of basic red dye. Int J Biol Macromol 120:1508–1514
Eldin MS, El-Sakka SA, El-Masry MM, Abdel-Gawad II, Garybe SS (2012) Removal of methylene blue dye from aqueous medium by nano poly acrylonitrile particles. Desalination Water Treat 44:151–160
Eldin MS, Aggour YA, El-Aassar MR, Beghet GE, Atta RR (2016) Development of nano-crosslinked polyacrylonitrile ions exchanger particles for dyes removal. Desalination Water Treat 57(9):4255–4266
Elkady M, Mahmoud M, Abd-El-Rahman H (2011) Kinetic approach for cadmium sorption using microwave synthesized nano-hydroxyapatite. J NonCryst Solids 357:1118–1129
Elkady MF, El-Aassar MR, Hassan HS (2016) Adsorption profile of basic dye onto novel fabricated carboxylated functionalized co-polymer nanofibers. Polymers 8:177
El-Latif MMA, Elkady MF (2010) Equilibrium isotherms for harmful ions sorption using nano zirconium vanadate ion exchanger. Desalination 255:21–43
El-Latif MA, Ibrahim AM, El-Kady M (2010) Adsorption equilibrium, kinetics and thermodynamics of methylene blue from aqueous solutions using biopolymer oak sawdust composite. J Am Sci 6:267–283
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–470
Goldberg S (2005) Equations and models describing adsorption processes in soils. In: Chemical processes in soils. SSSA Book Series no. 8. Soil Science Society of America, Madison
Hashem F, Amin M (2014) Kinetic and thermal studies of removal of CrO42 − ions by ettringite. J Therm Anal Calorim 116:835–844
Hashem F, Amin M (2016) Adsorption of methylene blue by activated carbon derived from various fruit peels. Desalination Water Treat 57:22573–22584
Hassan HS, Elkady MF, El-Shazly AH, Bamufleh HS (2014) Formulation of Synthesized zinc oxide nanopowder into hybrid beads for dye separation. J Nanomater 2014:14
Jiuhui Q (2008) Research progress of novel adsorption processes in water purification: a review. J Environ Sci 20:1–13
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I. solids. J Am Chem Soc 38:2221–2295
McComb M, Gesser H (1997) Passive monitoring of trace metals in water by in situ sample preconcentration via chelation on a textile based solid sorbent. Anal Chim Acta 341:229–239
Memon GZ, Bhanger MI, Akhtar M, Talpur FN, Memon JR (2008) Adsorption of methyl parathion pesticide from water using watermelon peels as a low cost adsorbent. Chem Eng J 138:616–621
Miyaji F, Masuda S, Suyama Y (2010) Adsorption removal of lead and cadmium ions from aqueous solution with coal fly ash-derived zeolite/sepiolite composite. J Ceram Soc Jpn 118:1062–1066
Namasivayam C, Sureshkumar MV (2008) Removal of chromium (VI) from water and wastewater using surfactant modified coconut coir pith as a biosorbent. Biores Technol 99:2218–2225
Ozacar M, Sengil IA (2005) A kinetic study of metal complex dye sorption onto pine sawdust. Process Biochem 40:565–572
Plazinski W, Rudzinski W, Plazinska A (2009) Theoretical models of sorption kinetics including a surface reaction mechanism: a review. Adv Coll Interface Sci 152:2–13
Pourjavadi A, Hosseinzadeh H (2010) Synthesis and properties of partially hydrolyzed acrylonitrile-co-acrylamide superabsorbent hydrogel. Drugs 13:14
Ruixia L, Jinlong G, Hongxiao T (2002) Adsorption of fluoride, phosphate, and arsenate ions on a new type of ion exchange fiber. J Colloid Interface Sci 248:268–274
Shunkevich A, Akulich Z, Mediak G, Soldatov V (2005) Acid-base properties of ion exchangers. III. Anion exchangers on the basis of polyacrylonitrile fiber. React Funct Polym 63:27–34
Soldatov V, Shunkevich A, Elinson I, Johann J, Iraushek H (1999) Chemically active textile materials as efficient means for water purification. Desalination 124:181–192
Suteu D, Bilba D, Coseri S (2014) Macroporous polymeric ion exchangers as adsorbents for the removal of cationic dye basic blue 9 from aqueous solutions. J Appl Polym Sci 131:39620. https://doi.org/10.1002/app.39620
Temkin MJ, Pyzhev V (1940) Recent modifications to Langmuir isotherms. Acta Physiochim URSS 12:217–222
Vatutsina O, Soldatov V, Sokolova V, Johann J, Bissen M, Weissenbacher A (2007) A new hybrid (polymer/inorganic) fibrous sorbent for arsenic removal from drinking water. React Funct Polym 67:184–201
Acknowledgements
This work has been supported by City of Scientific Research and Technological Applications (SRTA-city). We report no conflicts of interest in this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: M. Abbaspour.
Rights and permissions
About this article
Cite this article
El-Aassar, M.R., Hassan, H.S., Elkady, M.F. et al. Isothermal, kinetic, and thermodynamic studies on copper adsorption on modified styrene acrylonitrile copolymer. Int. J. Environ. Sci. Technol. 16, 7037–7048 (2019). https://doi.org/10.1007/s13762-018-02199-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-018-02199-x