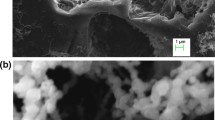
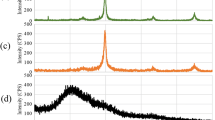
Abstract
In this study, sepiolite-nano zero valent iron composite was synthesized and applied for its potential adsorption to remove phosphates from aqueous solution. This composite was characterized by different techniques. For optimization of independent parameters (pH = 3–9; initial phosphate concentration = 5–100 mg/L; adsorbent dosage = 0.2–1 g/L; and contact time = 5–100 min), response surface methodology based on central composite design was used. Adsorption isotherms and kinetic models were done under optimum conditions. The results indicated that maximum adsorption efficiency of 99.43 and 92% for synthetic solution and real surface water sample, respectively, were achieved at optimum conditions of pH 4.5, initial phosphate concentration of 25 mg/L, adsorbent dosage of 0.8 g/L, and 46.26 min contact time. The interaction between adsorbent and adsorbate is better described with the Freundlich isotherm (R 2 = 0.9537), and the kinetic of adsorption process followed pseudo-second-order model. Electrostatic interaction was the major mechanisms of the removal of phosphates from aqueous solution. The findings of this study showed that there is an effective adsorbent for removal of phosphates from aqueous solutions.






Similar content being viewed by others
References
Al-Ghouti MA, Khraisheh MA, Ahmad MN, Allen S (2009) Adsorption behaviour of methylene blue onto Jordanian diatomite: a kinetic study. J Hazard Mater 165:589–598. doi:10.1016/j.hazmat.2008.0.018
Almeelbi T, Bezbaruah A (2012) Aqueous phosphate removal using nanoscale zero-valent iron. J Nanopart Res 14:1–14. doi:10.1007/s11051-012-0900-y
Arshadi M, Soleymanzadeh M, Salvacion J, Salimivahid F (2014) Nanoscale zero-valent iron (NZVI) supported on sineguelas waste for Pb(II) removal from aqueous solution: kinetics, thermodynamic and mechanism. J Colloid Interf Sci 426:241–251. doi:10.1016/j.jcis.2014.04.014
Arshadi M, Foroughifard S, Gholtash JE, Abbaspourrad A (2015) Preparation of iron nanoparticles-loaded Spondias purpurea seed waste as an excellent adsorbent for removal of phosphate from synthetic and natural waters. J Colloid Interf Sci 452:69–77. doi:10.1016/j.jcis.2015.04.019
Asfaram A, Fathi M, Khodadoust S, Naraki M (2014) Removal of Direct Red 12B by garlic peel as a cheap adsorbent: kinetics, thermodynamic and equilibrium isotherms study of removal. Spectrochim Acta A 127:415–421. doi:10.1016/j.saa.2014.02.092
Bakhtiary S, Shirvani M, Shariatmadari H (2013) Characterization and 2, 4-D adsorption of sepiolite nanofibers modified by N-cetylpyridinium cations. Microporous Mesoporous Mat 168:30–36. doi:10.1016/j.micromeso.2012.09.022
Berkani M, Bouhelassa M, Bouchareb MK (2015) Implementation of a venturi photocatalytic reactor: optimization of photodecolorization of an industrial azo dye. Arab J Chem. doi:10.1016/j.arabjc.2015.07.004
Chakraborty S, Dasgupta J, Farooq U, Sikder J, Drioli E, Curcio S (2014) Experimental analysis, modeling and optimization of chromium (VI) removal from aqueous solutions by polymer-enhanced ultrafiltration. J Membr Sci 456:139–154. doi:10.1016/j.memsci.2014.01.016
Chen ZX, Jin XY, Chen Z, Megharaj M, Naidu R (2011) Removal of methyl orange from aqueous solution using bentonite-supported nanoscale zero-valent iron. J Colloid Interf Sci 363:601–607. doi:10.1016/j.jcis.2011.07.057
Choi JW, Choi YS, Hong SW, Kim DJ, Lee SH (2012) Effect of pH and coexisting anions on removal of phosphate from aqueous solutions by inorganic-based mesostructures. Water Environ Res 84:596–604. doi:10.2175/106143012X13373575830755
Clesceri L, Greenberg A, Eaton A (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, DC (variously paged)
Dai L, Wu B, Tan F, He M, Wang W, Qin H, Tang X, Zhu Q, Pan K, Hu Q (2014) Engineered hydrochar composites for phosphorus removal/recovery: lanthanum doped hydrochar prepared by hydrothermal carbonization of lanthanum pretreated rice straw. Biores Technol 161:327–332. doi:10.1016/j.biortech.2014.03.086
Dai Y, Hu Y, Jiang B, Zou J, Tian G, Fu H (2015) Carbothermal synthesis of ordered mesoporous carbon-supported nano zero-valent iron with enhanced stability and activity for hexavalent chromium reduction. J Hazard Mater 309:249–258. doi:10.1016/j.jhazmat.2015.04.013
Daneshkhah M, Hossaini H, Malakootian M (2017) Removal of metoprolol from water by sepiolite-supported nanoscale zero-valent iron. J Environ Chem Eng 5:3490–3499. doi:10.1016/j.jece.2017.06.040
Dasgupta J, Singh M, Sikder J, Padarthi V, Chakraborty S, Curcio S (2015) Response surface-optimized removal of Reactive Red 120 dye from its aqueous solutions using polyethyleneimine enhanced ultrafiltration. Ecotox Environ Safe 121:271–278. doi:10.1016/j.ecoenv.2014.12.041
Esfahani AR, Hojati S, Azimi A, Farzadian M, Khataee A (2014) Enhanced hexavalent chromium removal from aqueous solution using a sepiolite-stabilized zero-valent iron nanocomposite: impact of operational parameters and artificial neural network modeling. J Taiwan Inst Chem Eng 49:172–182. doi:10.1016/j.jtice.2014.11.011
Fateminia FS, Falamaki C (2013) Zero valent nano-sized iron/clinoptilolite modified with zero valent copper for reductive nitrate removal. Process Saf Environ 91:304–310. doi:10.1016/j.psep.2012.07.005
Fiedor JN, Bostick WD, Jarabek RJ, Farrell J (1998) Understanding the mechanism of uranium removal from groundwater by zero-valent iron using X-ray photoelectron spectroscopy. Environ Sci Technol 32:1466–1473
Fu R, Yang Y, Xu Z, Zhang X, Guo X, Bi D (2015) The removal of chromium (VI) and lead (II) from groundwater using sepiolite-supported nanoscale zero-valent iron (S-NZVI). Chemosphere 138:726–734. doi:10.1016/j.chemosphere.2015.07.051
Geng B, Jin Z, Li T, Qi X (2009) Kinetics of hexavalent chromium removal from water by chitosan-Fe 0 nanoparticles. Chemosphere 75:825–830. doi:10.1016/j.chemosphere.2009.01.009
Gök Ö, Özcan AS, Özcan A (2008) Adsorption kinetics of naphthalene onto organo-sepiolite from aqueous solutions. Desalination 220:96–107. doi:10.1016/j.desal.2007.01.025
Hassani A, Soltani RDC, Karaca S, Khataee A (2015) Preparation of montmorillonite–alginate nanobiocomposite for adsorption of a textile dye in aqueous phase: isotherm, kinetic and experimental design approaches. J Ind Eng Chem 21:1197–1207. doi:10.1016/j.jiec.2014.05.034
Kim SA, Kamala-Kannan S, Lee KJ, Park YJ, Shea PJ, Lee WH, Kim HM, Oh BT (2013) Removal of Pb(II) from aqueous solution by a zeolite–nanoscale zero-valent iron composite. Chem Eng J 217:54–60. doi:10.1016/j.cej.2012.11.097
Lescano L, Castillo L, Marfil S, Barbosa S, Maiza P (2014) Alternative methodologies for sepiolite defibering. Appl Clay Sci 95:378–382. doi:10.1016/j.clay.2014.05.001
Li G, Gao S, Zhang G, Zhang X (2014) Enhanced adsorption of phosphate from aqueous solution by nanostructured iron (III)–copper (II) binary oxides. Chem Eng J 235:124–131. doi:10.1016/j.cej.203.09.021
Liu X, Zhang L (2015) Removal of phosphate anions using the modified chitosan beads: adsorption kinetic, isotherm and mechanism studies. Powder Technol 277:112–119. doi:10.1016/j.powtec.2015.02.055
Liu H, Chen T, Zou X, Xie Q, Qing C, Chen D, Frost RL (2013) Removal of phosphorus using NZVI derived from reducing natural goethite. Chem Eng J 234:80–87. doi:10.1016/j.cej.2013.08.061
Liu F, Yang J, Zuo J, Ma D, Gan L, Xie B, Wang P, Yang B (2014a) Graphene-supported nanoscale zero-valent iron: removal of phosphorus from aqueous solution and mechanistic study. J Environ Sci 26:1751–1762. doi:10.1016/j.jes.2014.06.016
Liu T, Wang ZL, Yan X, Zhang B (2014b) Removal of mercury (II) and chromium (VI) from wastewater using a new and effective composite: pumice-supported nanoscale zero-valent iron. Chem Eng J 245:34–40. doi:10.1016/j.cej.2014.02.011
Lu S, Bai S, Zhu L, Shan H (2009) Removal mechanism of phosphate from aqueous solution by fly ash. J Hazard Mater 161:95–101. doi:10.1016/j.jhazmat.2008.02.123
Lu J, Liu D, Hao J, Zhang G, Lu B (2015) Phosphate removal from aqueous solutions by a nano-structured Fe–Ti bimetal oxide sorbent. Chem Eng Res Des 93:652–661. doi:10.1016/j.cherd.2014.05.001
Luo S, Qin P, Shao J, Peng L, Zeng Q, Gu JD (2013) Synthesis of reactive nanoscale zero valent iron using rectorite supports and its application for Orange II removal. Chem Eng J 223:1–7. doi:10.1016/j.cej.2012.10.088
Malakootian M, Yousefi N, Jaafarzadeh HN (2011) Kinetics modeling and isotherms for adsorption of phosphate from aqueous solution by modified clinoptilolite. Water Wastewater 22:21–22
Malakootian M, Javdan M, Iranmanesh F (2015a) Fluoride removal study from aqueous solutions using Jajarm bauxite: case study on Koohbanan water. Fluoride 48:113–122
Malakootian M, Mansoorian HJ, Hosseini A, Khanjani N (2015b) Evaluating the efficacy of alumina/carbon nanotube hybrid adsorbents in removing azo Reactive Red 198 and Blue 19 dyes from aqueous solutions. Process saf Environ 96:125–137. doi:10.1016/j.psep.2015.05.002
Malakootian M, Nori Sepehr M, BahrainiS Zarrabi M (2016a) Capacity of natural and modified zeolite with cationic surfactant in removal of antibiotic tetracycline from aqueous solutions. Koomesh 17:779–788
Malakootian M, Pourshaban-Mazandarani M, Hossaini H, Ehrampoush MH (2016b) Preparation and characterization of TiO2 incorporated 13X molecular sieves for photocatalytic removal of acetaminophen from aqueous solutions. Process saf Environ 104:334–345. doi:10.1016/j.psep.2016.09.018
Malakootian M, Ehrampoush MH, Hossaini H, Pourshaban-Mazandarani M (2016c) Acetaminophen removal from Aqueous Solutions by TiO2-X photo catalyst. Tolooebehdasht 14:200–213
Moussavi G, Hosseini H, Alahabadi A (2013) The investigation of diazinon pesticide removal from contaminated water by adsorption onto NH4 Cl-induced activated carbon. Chem Eng J 214:172–179. doi:10.1016/j.cej.2012.10.034
O’carroll D, Sleep B, Krol M, Boparai H, Kocur C (2013) Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv Water Resour 51:104–122. doi:10.1016/j.advwatres.2012.02.005
Olya M, Vafaee M, Jahangiri M (2015) Modeling of acid dye decolorization by TiO2–Ag2O nano-photocatalytic process using response surface methodology. J Saudi Chem Soc. doi:10.1016/j.jscs.2015.07.006
Özcan AS, Gök Ö (2012) Structural characterization of dodecyltrimethylammonium (DTMA) bromide modified sepiolite and its adsorption isotherm studies. J Mol Struct 1007:36–44. doi:10.1016/j.molstruc.2011.09.044
Perraki T, Orfanoudaki A (2008) Study of raw and thermally treated sepiolite from the Mantoudi area, Euboea, Greece. J Therm Anal Calorim 91:589–593. doi:10.1007/s10973-007-8329-8
Qiu L, Wang G, Zhang S, Huang K (2014) Phosphate removal through crystallization using hydrothermal modified steel slag-based material as seed crystal. Desalin Water Treat 52:384–389. doi:10.1080/19443994.2013.795725
Qiu L, Zheng P, Zhang M, Yu X, Abbas G (2015) Phosphorus removal using ferric–calcium complex as precipitant: parameters optimization and phosphorus-recycling potential. Chem Eng J 268:230–235. doi:10.1016/j.cej.2014.12.107
Ragheb SM (2013) Phosphate removal from aqueous solution using slag and fly ash. HBRC J 9:270–275. doi:10.1016/j.hbrcj.2013.08.005
Shu HY, Chang MC, Chen CC, Chen PE (2010) Using resin supported nano zero-valent iron particles for decoloration of Acid Blue 113 azo dye solution. J Hazard Mater 184:499–505. doi:10.1016/j.jhazmat.2010.08.064
Singh P, Raizada P, Kumari S, Kumar A, Pathania D, Thakur P (2014) Solar-Fenton removal of malachite green with novel Fe0-activated carbon nanocomposite. Appl Catal A-Gen 476:9–18. doi:10.1016/j.apcata.2014.02.009
Soylemez S, Kanik FE, Tarkuc S, Udum YA, Toppare L (2013) A sepiolite modified conducting polymer based biosensor. Colloid Surf B 111:549–555. doi:10.1016/j.colsurfb.2013.07.013
Su Y, Cui H, Li Q, Gao S, Shang JK (2013) Strong adsorption of phosphate by amorphous zirconium oxide nanoparticles. Water Res 47:5018–5026. doi:10.1016/j.watres.2013.05.044
Su Y, Yang W, Sun W, Li Q, Shang JK (2015) Synthesis of mesoporous cerium–zirconium binary oxide nanoadsorbents by a solvothermal process and their effective adsorption of phosphate from water. Chem Eng J 268:270–279. doi:10.1016/j.cej.2015.01.070
Suárez M, García-romero E (2012) Variability of the surface properties of sepiolite. Appl Clay Sci 67:72–82. doi:10.1016/j.clay.2012.06.003
Sun Y, Wang Q, Yang S, Sheng G, Guo Z (2011) Characterization of nano-iron oxyhydroxides and their application in UO2 2+ removal from aqueous solutions. J Radioanal Nucl Chem 290:643–648. doi:10.1007/s10967-011-1325-2
Sun Z, Zheng S, Ayoko GA, Frost RL, Xi Y (2013) Degradation of simazine from aqueous solutions by diatomite-supported nanosized zero-valent iron composite materials. J Hazard Mater 263:768–777. doi:10.1016/j.jhazmat.2013.10.045
Sun X, Yan Y, Li J, Han W, Wang L (2014) SBA-15-incorporated nanoscale zero-valent iron particles for chromium (VI) removal from groundwater: mechanism, effect of pH, humic acid and sustained reactivity. J Hazard Mater 266:26–33. doi:10.1016/j.jhazmat.2013.12.001
Tunc S, Duman O, Kancı B (2012) Rheological measurements of Na-bentonite and sepiolite particles in the presence of tetradecyltrimethylammonium bromide, sodium tetradecyl sulfonate and Brij 30 surfactants. Colloid Surf A 398:37–47. doi:10.1016/j.colsurfa.2012.02.006
Üzum Ç, Shahwan T, Eroğlu AE, Hallam KR, Scott TB, Lieberwirth I (2009) Synthesis and characterization of kaolinite-supported zero-valent iron nanoparticles and their application for the removal of aqueous Cu2+ and Co2+ ions. Appl Clay Sci 43:172–181. doi:10.1016/j.clay.2008.07.030
Wang W, Zhang H, Zhang L, Wan H, Zheng S, Xu Z (2015) Adsorptive removal of phosphate by magnetic Fe3 O4@ C@ ZrO2. Colloid Surf A 469:100–106. doi:10.1016/j.colsurfa.2015.01.002
Woumfo ED, Siewe JM, Njopwouo D (2015) A fixed-bed column for phosphate removal from aqueous solutions using an andosol-bagasse mixture. J Environ Manag 151:450–460. doi:10.1016/j.jenvman.2014.11.029
Xiao J, Gao B, Yue Q, Gao Y, Li Q (2015a) Removal of trihalomethanes from reclaimed-water by original and modified nanoscale zero-valent iron: characterization, kinetics and mechanism. Chem Eng J 262:1226–1236. doi:10.1016/j.cej.2014.10.080
Xiao L, Xiong Y, Tian S, He C, Su Q, Wen Z (2015b) One-dimensional coordination supramolecular polymer [Cu (bipy)(SO4)] n as an adsorbent for adsorption and kinetic separation of anionic dyes. Chem Eng J 265:157–163. doi:10.1016/j.cej.2014.11.134
Xie J, Lin Y, Li C, Wu D, Kong H (2015) Removal and recovery of phosphate from water by activated aluminum oxide and lanthanum oxide. Powder Technol 269:351–357. doi:10.1016/j.powtec.2014.09.024
Yan Y, Sun X, Ma F, Li J, Shen J, Han W, Liu X, Wang L (2014) Removal of phosphate from wastewater using alkaline residue. J Environ Sci 26:970–980. doi:10.1016/S1001-0742(13)60537-9
Yao Y, Gao B, Chen J, Yang L (2013) Engineered biochar reclaiming phosphate from aqueous solutions: mechanisms and potential application as a slow-release fertilizer. Environ Sci Technol 47:8700–8708. doi:10.1021/es4012977
Ye J, Cong X, Zhang P, Zeng G, Hoffmann E, Liu Y, Wu Y, Zhang H, Fang W, Hahn HH (2016) Application of acid-activated Bauxsol for wastewater treatment with high phosphate concentration: characterization, adsorption optimization, and desorption behaviors. J Environ Manag 167:1–7. doi:10.1016/j.jenvman.2015.ll.023
Yin H, Yun Y, Zhang Y, Fan C (2011) Phosphate removal from wastewaters by a naturally occurring, calcium-rich sepiolite. J Hazard Mater 198:362–369. doi:10.1016/j.jhazmat.2011.10.072
Yoshino H, Tokumura M, Kawase Y (2014) Simultaneous removal of nitrate, hydrogen peroxide and phosphate in semiconductor acidic wastewater by zero-valent iron. J Environ Sci Heal A 49:998–1006. doi:10.1080/10934529.2014.894841
Yu Y, Chen JP (2015) Key factors for optimum performance in phosphate removal from contaminated water by a Fe–Mg–La tri-metal composite sorbent. J Colloid Interf Sci 445:303–311. doi:10.1016/j.jcis.2014.12.056
Zhang G, Liu H, Liu R, Qu J (2009) Removal of phosphate from water by a Fe–Mn binary oxide adsorbent. J Colloid Interf Sci 335:168–174. doi:10.1016/j.jcis.2009.03.019
Zhang Y, Wang L, Lu D, Shi X, Wang C, Duan X (2012) Sensitive determination of bisphenol A base on arginine functionalized nanocomposite graphene film. Electrochim Acta 80:77–83. doi:10.1016/j.electacta.2012.06.117
Zhu Y, Zhu Z, Chen Y, Yang F, Qin H, Xie L (2013) Kinetics and thermodynamics of sorption for as (V) on the porous biomorph-genetic composite of α-Fe2O3/Fe3O4/C with eucalyptus wood hierarchical microstructure. Water Air Soil Pollut 224:1–19. doi:10.1016/j.seppur.2013.05.048
Zong E, Wei D, Wan H, Zheng S, Xu Z, Zhu D (2013) Adsorptive removal of phosphate ions from aqueous solution using zirconia-functionalized graphite oxide. Chem Eng J 221: 193-203. DOI: http://dx.doi.org/!0.1016/j.cej.2013.01.088
Acknowledgements
This research was conducted at the Environmental Health Engineering Research Center and was sponsored by the Vice-Chancellor for Research and Technology of Kerman University of Medical Sciences. The authors take this opportunity to express their gratitude for the support and assistance extended by the facilitators during the conduct of the research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Hari Pant.
Rights and permissions
About this article
Cite this article
Malakootian, M., Daneshkhah, M. & Hossaini, H. Removal of phosphates from aqueous solution by sepiolite-nano zero valent iron composite optimization with response surface methodology. Int. J. Environ. Sci. Technol. 15, 2129–2140 (2018). https://doi.org/10.1007/s13762-017-1520-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-017-1520-y