Abstract
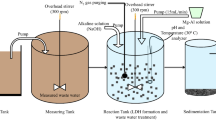
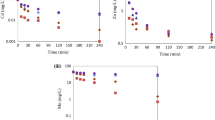
Health hazards from heavy metal pollution in water systems are a global environmental problem. Of similar concern is sludge that results from wastewater treatment due to unsatisfactory sludge management technology. Therefore, the effectiveness of using Mg–Al-layered double hydroxide in the removal of heavy metals from mine wastewater was tested and compared with that of calcium hydroxide [Ca(OH)2], which is a common treatment method for heavy metal removal. Initially, the mine wastewater contained cations of the heavy metals iron (Fe), zinc (Zn), copper (Cu), and lead (Pb). The Mg–Al-layered double hydroxides were able to remove 371, 7.2, 121, and 0.4 mg/L of these pollutants, respectively, using the co-precipitation method. The removal of these metals is most effective using 0.5 g Mg–Al-layered double hydroxide (Mg/Al molar ratio 4) and 20 min of shaking. Zn was removed by the formation of Zn(NO3)(OH)·H2O and Zn5(NO3)2(OH)8 when LDH, Mg/Al molar ratios of 4 and 2, respectively, were used. Similarly, Fe, Cu, and Pb were removed by the formation of Fe–Al-layered double hydroxide, Cu2(OH)3·NO3 and Pb4(OH)4(NO3)4, respectively. While Ca(OH)2 is also capable of reducing the heavy metal concentrations below the Japanese recommended values, this analysis shows that using 0.5 g Mg–Al-layered double hydroxide is a better treatment condition for mine wastewater, because it generates lower sludge volumes than 0.1 g of Ca(OH)2. The measured sludge volume was 1.5 mL for Mg–Al-layered double hydroxide and 2.5 mL for Ca(OH)2, a nearly twofold further reduction.








Similar content being viewed by others
References
Cavani F, Trifiro F, Vaccari A (1991) Hydrotalcite-type anionic clays: preparation, properties and applications. Catal Today 11:173–301
Cuerda-Correa EM, Gomez-Tamayo MD, Macias-Garcia A, Diez MAD (2008) Adsorption of Zn(II) in aqueous solution by activated carbons prepared from evergreen oak (Quercus rotundifolia L.). J Hazard Mater 153:28–36
Cursino ACT, Lisboa FS, Pyrrho AS, Sousa VP, Wypych F (2013) Layered double hydroxides intercalated with anionic surfactants/benzophenone as potential materials for sunscreens. J Colloid Interface Sci 397:88–95
Doan HD, Dang VBH, Dang-Vu T, Lohi A (2009) Equilibrium and kinetics of biosorption of cadmium(II) and copper(II) ions by wheat straw. Bioresour Technol 100:211–219
Evans DG, Slade RCT (2005) Structural aspects of layered double hydroxides. Struct Bonding (Berlin) 119:1–87
Goel J, Kadirvelu K, Rajagopal C, Garg VK (2005) Removal of lead(II) by adsorption using treated granular activated carbon: batch and column studies. J Hazard Mater 125(1–3):211–220
Grimes SM, Johnston SR, Abrahams I (1995) Characterisation of the predominant low-pH Lead(II)-Hydroxo cation, [Pb4(OH)4]4+; crystal structure of [Pb4(OH)4][NO3]4 and the implications of basic salt formation on the transport of lead in the aqueous environment. J Chem Soc Dalton Trans. doi:10.1039/DT9950002081
Grover K, Komarneni S, Katsuki H (2009) Uptake of arsenite by synthetic layered double hydroxides. Water Res 43:3884–3890
Guo XY, Liang S, Tian QH (2011) Adsorption of Pb2+ and Zn2+ from aqueous solutions by sulfured orange peel. Desalination 275:212–216
Halajnia A, Oustan S, Najafi N, Khataee AR, Lakzian A (2013) Adsorption-desorption characteristics of nitrate, phosphate and sulfate on Mg-Al layered double hydroxide. Appl Clay Sci 80–81:305–312
Kameda T, Takeuchi H, Yoshioka T (2010) Kinetics of uptake of Cu2+ and Cd2+ by Mg–Al layered double hydroxides intercalated with citrate, malate, and tartrate. Colloids Surf A Physicochem Eng Asp 355:172–177
Kameda T, Nakamura M, Yoshioka T (2012) Removal of antimonite ions from an aqueous solution by anion exchange with magnesium–aluminum layered double hydroxide and the formation of a brandholzite-like structure. J Environ Sci Health A 47:1146–1151
Kameda T, Kondo E, Yoshioka T (2014) Preparation of Mg–Al layered double hydroxide doped with Fe2+ and its application to Cr(VI) removal. Sep Purif Technol 122:12–16
Kameda T, Kondo E, Yoshioka T (2015) Equibrium and kinetics studies on As(V) and Sb(V) removal by Fe2+-doped Mg–Al layered double hydroxides. J Environ Manag 151:303–309
Komarneni S, Kozai N, Roy R (1998) Novel function for anionic clays: selective transition metal cation uptake by diodochy. J Mater Chem 8(6):1329–1331
Liu R, Frost RL, Martens WN (2009) Absorption of the selenite anion from aqueous solutions by thermally activated layered double hydroxide. Water Res 43:1323–1329
Louer PM, Grandjean DLD (1973) Etude Structurale des Hydroxynitrates de Nickel et de Zinc. III. Etude Structurale des Nitrates Basiques Zn(OH)2. Zn(NO3)2·2H2O et Ni(OH)2. Ni(NO3)2·2H2O. Acta Cryst B29:1707–1710
Park M, Choi CL, Seo YJ, Yeo SK, Choi J, Komarneni S, Lee JH (2007) Reaction of Cu2+ and Pb2+ with Mg/Al layered double hydroxide. Appl Clay Sci 37:143–148
Potera C (2004) Copper in drinking water: using symptoms of exposure to define safety. Environ Health Perspect 112(10):A568–A569
Rahman MT, Kameda T, Kumagai S, Yoshioka T (2016a) Adsorption isotherm and kinetics of arsenic removal from aqueous solution by Mg–Al layered double hydroxide intercalated with nitrate ions. Reac Kinet Mech Cat. doi:10.1007/s11144-016-1116-4
Rahman MT, Kameda T, Kumagai S, Yoshioka T (2016b) Application of Mg–Al layered double hydroxide for removing heavy metals from mine waste water: a novel approach to reduction of sludge. Mine Water Environ (under review)
Sadik R, Lahkale R, Hssaine N, ElHatimi W, Diouri M, Sabbar E (2015) Sulfate removal from wastewater by mixed oxide-LDH: equilibrium, kinetic and thermodynamic studies. J Mater Environ Sci 6(10):2895–2905
Segalen C, Dieude-Fauvel E, Baudez JC (2015) Electrical and rheological properties of sewage sludge—Impact of the solid content. Water Res 82:25–36
Shan R, Yan L, Yang K, Hao Y, Du B (2015) Adsorption of Cd(II) by Mg–Al–CO3 and magnetic Fe3O4/Mg–Al–CO3 layered double hydroxides: kinetic, isothermal, thermodynamic and mechanistic studies. J Hazard Mater 299:42–49
Sheoran AS, Sheoran V (2006) Heavy metal removal mechanism of acid mine drainage in wetlands: a critical review. Miner Eng 19:105–116
Sideris PJ, Nielsen UG, Gan ZH, Grey CP (2008) Mg/Al ordering in layered double hydroxides revealed by multinuclear NMR spectroscopy. Science 321:113–117
Wang SL, Liu CH, Wang MK, Chuang YH, Chiang PN (2009) Arsenate adsorption by Mg/Al–NO3 layered double hydroxides with varying the Mg/Al ratio. Appl Clay Sci 43:79–85
You Y, Vance GF, Zhao H (2001) Selenium adsorption on Mg–Al and Zn–Al layered double hydroxides. Appl Clay Sci 20:13–25
Yousuf I (2013) Methods for estimation and comparison of activated sludge settleability. 38th Annual WIOA Qld water industry operations conference, Parklands, Gold Coast
Zhang S (2014) A new nano-sized calcium hydroxide photocatalytic material for the photodegradation of organic dyes. RSC Adv 4:15835–15840
Acknowledgements
The authors are grateful to the financial support from the Japan Oil, Gas and Metals Nationals Corporations (JOGMEC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Binbin Huang.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rahman, M.T., Kameda, T., Kumagai, S. et al. Effectiveness of Mg–Al-layered double hydroxide for heavy metal removal from mine wastewater and sludge volume reduction. Int. J. Environ. Sci. Technol. 15, 263–272 (2018). https://doi.org/10.1007/s13762-017-1385-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-017-1385-0