Abstract
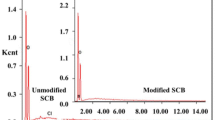
Tetraethylenepentamine-modified sugarcane bagasse (SCB) was prepared to improve its adsorption capacity and selectivity toward Cu2+. Adsorption performances of the modified sorbent for Cu2+ were studied in batch system. Separation of Cu2+ from Pb2+ by the modified sorbent fixed-bed column were studied under dynamic system with initial molar concentration ratio \(\left( {C_{0}^{\text{Cu}} /C_{0}^{\text{Pb}} } \right)\) ranging from 1:1 to 1:100. The amount of Cu2+ and Pb2+ adsorbed on the saturated column was calculated by the elution curve. Batch experimental results showed that the adsorption capacity of the sorbent for Cu2+ increased from 0.12 to 0.21 mmol g−1 after modification. Dynamic adsorption results showed that the modified SCB had higher adsorption affinity toward Cu2+ than Pb2+. 0.07 mmol g−1 of adsorbed Pb2+ was pushed off by Cu2+ during the competitive adsorption process at \(C_{0}^{\text{Cu}} /C_{0}^{\text{Pb}} = {\text{1:1}}.\) The breakthrough curves and adsorption kinetics of Cu2+ in the column could be fitted well by the Yoon–Nelson and modified Yoon–Nelson model, respectively. According to the elution curve, the amount of Cu2+ adsorbed on the fixed-bed column were 0.16, 0.16 and 0.15 mmol g−1, while that of Pb2+ were 0.0016, 0.0051 and 0.0094 mmol g−1 when \(C_{0}^{\text{Cu}} /C_{0}^{\text{Pb}}\) increased from 1:1 to 1:10 and 1:100. Cu2+ could be selectively adsorbed and separated from Pb2+ by using the modified sorbent fixed-bed column.








Similar content being viewed by others
References
Bayo J (2012) Kinetic studies for Cd(II) biosorption from treated urban effluents by native grape fruit biomass (Citrus paradisi L.): The competitive effect of Pb(II), Cu(II) and Ni(II). Chem Eng J 191:278–287
Deng SB, Ting YP (2005) Characterization of PEI-modified biomass and biosorption of Cu(II), Pb(II) and Ni(II). Water Res 39:2167–2177
Deng YH, Gao ZQ, Liu BZ, Hu XB, Wei ZB, Sun C (2013) Selective removal of lead from aqueous solutions by ethylenediamine modified attapulgite. Chem Eng J 223:91–98
Escudero C, Poch J, Villaescusa I (2013) Modelling of breakthrough curves of single and binary mixtures of Cu(II), Cd(II), Ni(II) and Pb(II) sorption onto grape stalks waste. Chem Eng J 217:129–138
Goyal P, Srivastava S (2009) Characterization of novel Zea mays based biomaterial designed for toxic metals biosorption. J Hazard Mater 172:1206–1211
Hu X, Zhao M, Song G, Huang H (2011) Modification of pineapple peel fiber with succinic anhydride for Cu(II), Cd(II) and Pb(II) removal from aqueous solutions. Environ Technol 32:739–746
Isaac RA, Gil L, Cooperman AN, Hulme K, Eddy B, Ruiz M, Jacobson K, Larson C, Pancorbo OC (1997) Corrosion in drinking water distribution systems: a major contributor of copper and lead to wastewaters and effluents. Environ Sci Technol 31:3198–3203
Kratochvil D, Volesky B (1998) Advances in the biosorption of heavy metals. Trends Biotechnol 16:291–300
Krishnan KA, Sreejalekshmi KG, Baiju RS (2011) Nickel(II) adsorption onto biomass based activated carbon obtained from sugarcane bagasse pith. Bioresour Technol 102:10239–10247
Krishnan KA, Sreejalekshmi KG, Vimexen V, Dev Vinu V (2016) Evaluation of adsorption properties of sulphurised activated carbon for the effective and economically viable removal of Zn(II) from aqueous solutions. Ecotoxicol Environ Saf 124:418–425
Kuang SP, Wang ZZ, Liu J, Wu ZC (2013) Preparation of triethylene-tetramine grafted magnetic chitosan for adsorption of Pb(II) ion from aqueous solutions. J Hazard Mater 260:210–219
Liu CK, Bai RB, Hong L, Liu T (2010) Functionalization of adsorbent with different aliphatic polyamines for heavy metal ion removal: characteristics and performance. J Colloid Interface Sci 345:454–460
Madrid JF, Nuesca GM, Abad LV (2014) Amine functionalized radiation-induced grafted water hyacinth fibers for Pb2+, Cu2+ and Cr2+ uptake. Radiat Phys Chem 97:246–252
Motsa MM, Mamba BB, Thwala JM, Msagati TAM (2011) Preparation, characterization, and application of polypropylene–clinoptilolite composites for the selective adsorption of lead from aqueous media. J Colloid Interface Sci 359:210–219
Naiya TK, Bhattacharya AK, Mandal S, Das SK (2009) The sorption of lead(II) ions on rice husk ash. J Hazard Mater 163:1254–1264
Nemr AE, El-Sikaily A, Khaled A, Abdelwahab O (2015) Removal of toxic chromium from aqueous solution, wastewater and saline water by marine red alga Pterocladiacapillacea and its activated carbon. Arab J Chem 8:105–117
Nguyen TAH, Ngo HH, Guo WS, Zhang J, Liang S, Yue QY, Li Q, Nguyen TV (2013) Corrosion in drinking water distribution systems: a major contributor of copper and lead to wastewaters and effluents. Bioresour Technol 148:574–585
Nuić I, Trgo M, Perić J, Medvidovic NV (2013) Analysis of breakthrough curves of Pb and Zn sorption from binary solutions on natural clinoptilolite. Micropor Mesopor Mater 167:55–61
Qaiser S, Saleemi AR, Umar M (2009) Biosorption of lead from aqueous solution by Ficus religiosa leaves: Batch and column study. J Hazard Mater 166:998–1005
Rosales E, Ferreira L, Sanromán M, Tavares T, Pazos M (2015) Enhanced selective metal adsorption on optimised agroforestry waste mixtures. Bioresour Technol 182:41–49
Sag Y, Kutsal T (2001) Recent trends in the biosorption of heavy metals: a review. Biotechnol Bioprocess Eng 6:376–385
Shen HY, Pan SD, Zhang Y, Huang XL, Gong HX (2012) A new insight on the adsorption mechanism of amino-functionalized nano-Fe3O4magnetic polymers in Cu(II), Cr(VI) co-existing water system. Chem Eng J 183:180–191
Simate GS, Ndlovu S (2015) The removal of heavy metals in a packed bed column using immobilized cassava peel waste biomass. J Ind Eng Chem 21:635–643
Xu D, Tan XL, Chen CL, Wang XK (2008) Adsorption of Pb(II) from aqueous solution to MX-80 bentonite: effect of pH, ionic strength, foreign ions and temperature. Appl Clay Sci 41:37–46
Xu MY, Yin P, Liu XG, Tang QH, Qu RJ, Xu Q (2013) Utilization of rice husks modified by organomultiphosphonic acids as low-cost biosorbents for enhanced adsorption of heavy metal ions. Bioresour Technol 149:420–424
Yu JX, Wang LY, Chi RA, Zhang YF, Xu ZG, Guo J (2015a) Adsorption of Pb2+, Cd2+, Cu2+ and Zn2+ from aqueous solution by modified sugarcane bagasse. Res Chem Intermed 41:1525–1541
Yu JX, Zhu J, Feng LY, Chi RA (2015b) Simultaneous removal of cationic and anionic dyes by the mixed sorbent of magnetic and non-magnetic modified sugarcane bagasse. J Colloid Interface Sci 451:153–160
Acknowledgments
The work is financially supported by National Natural Science Foundation of China (No. 51574182), the Key Project of Chinese Ministry of Education (No. 213024A) and the program for excellent young scientific and technological innovation team of Hubei Provincial Department of Education, China (No. T201506).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, J., Xiong, W., Zhu, J. et al. Separation of Cu2+ and Pb2+ by tetraethylenepentamine-modified sugarcane bagasse fixed-bed column: selective adsorption and kinetics. Int. J. Environ. Sci. Technol. 13, 1933–1940 (2016). https://doi.org/10.1007/s13762-016-1013-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-016-1013-4