Abstract
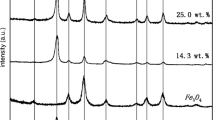
Fe3O4/multi-walled carbon nanotubes were prepared, characterized and used as a nanocatalyst for ozonation of p-hydroxybenzoic acid. The stability and reusability of the catalyst was evaluated. Characterization techniques including X-ray diffraction, Fourier transform infrared absorption spectroscopy, scanning electron microscope, high-resolution transmission electron microscopy and physical property measurement were used to analyze the reason for the decrease in catalyst activity. The addition of t-butanol and bicarbonate were used to explore the different process between hydroxyl radicals and ozone. The experimental results showed that the catalytic ozonation could significantly increase the degradation and mineralization of p-hydroxybenzoic acid. The initial pH value was a crucial factor influencing ozone decomposition and the surface property of catalyst or organic pollutant. The degradation of p-hydroxybenzoic acid increased by 32 % in catalyzed ozonation compared to single ozonation after 5 min reaction with unadjusted pH (about 5.4). In batch experiments, the removal efficiency of p-hydroxybenzoic acid and total organic carbon decreased 36.1 and 6.8 % after six run times. Bicarbonate significantly inhibited the mineralization of p-HBA, but it had almost no influence on the catalytic degradation of p-hydroxybenzoic acid. A possible pathway for p-hydroxybenzoic acid degradation was tentatively proposed.










Similar content being viewed by others
References
Beltrán FJ, Rivas FJ, Montero-de-Espinosa R (2003) Ozone-enhanced oxidation of oxalic acid in water with cobalt catalysts. 2. Heterogeneous catalytic ozonation. Ind Eng Chem Res 42(14):3218–3224
Buxton GV, Greenstock CL, Ross WPH, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O–) in aqueous solution. J Phys Chem Ref Data 17(2):513–886
Chen Y, Ai Z, Zhang L (2012) Enhanced decomposition of dimethyl phthalate via molecular oxygen activated by Fe@Fe2O3/AC under microwave irradiation. J Hazard Mater 235–236:92–100
Dai Q, Wang J, Yu J, Chen J, Chen J (2014) Catalytic ozonation for the degradation of acetylsalicylic acid in aqueous solution by magnetic CeO2 nanometer catalyst particles. Appl Catal B Environ 144:686–693
Deng J, Wen X, Wang Q (2012) Solvothermal in situ synthesis of Fe3O4-multi-walled carbon nanotubes with enhanced heterogeneous Fenton-like activity. Mater Res Bull 47(11):3369–3376
Duesterberg CK, Waite TD (2007) Kinetic modeling of the oxidation of p-hydroxybenzoic acid by Fenton’s reagent: implications of the role of quinones in the redox cycling of iron. Environ Sci Technol 41(11):4103–4110
Fan X, Restivo J, Órfão JJM, Pereira MFR, Lapkin AA (2014) The role of multiwalled carbon nanotubes (MWCNTs) in the catalytic ozonation of atrazine. Chem Eng J 241:66–76
Faria PCC, Monteiro DCM, Órfão JJM, Pereira MFR (2009) Cerium, manganese and cobalt oxides as catalysts for the ozonation of selected organic compounds. Chemosphere 74(6):818–824
Gharbani P, Tabatabaii SM, Mehrizad A (2008) Removal of Congo red from textile wastewater by ozonation. Int J Environ Sci Technol 4(5):495–500
Giri RR, Ozaki H, Ota S, Takanami R, Taniguchi S (2010) Degradation of common pharmaceuticals and personal care products in mixed solutions by advanced oxidation techniques. Int J Environ Sci Technol 7(2):251–260
Guo W, Yin R, Zhou X, Du J, Cao H, Yang S, Ren N (2015) Sulfamethoxazole degradation by ultrasound/ozone oxidation process in water: kinetics, mechanisms, and pathways. Ultrason Sonochem 22:182–187
Gupta VK, Nayak A (2012) Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. Chem Eng J 180:81–90
Gupta VK, Saleh TA (2013) Sorption of pollutants by porous carbon, carbon nanotubes and fullerene- an overview. Environ Sci Pollut Res 20(5):2828–2843
Gupta VK, Agarwal S, Saleh TA (2011a) Chromium removal by combining the magnetic properties of iron oxide with adsorption properties of carbon nanotubes. Water Res 45(6):2207–2212
Gupta VK, Agarwal S, Saleh TA (2011b) Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J Hazard Mater 185(1):17–23
Gupta VK, Ali I, Saleh TA, Nayak A, Agarwal S (2012a) Chemical treatment technologies for waste-water recycling—an overview. RSC Adv 2:6380–6388
Gupta VK, Jain R, Mittal A, Saleh TA, Nayak A, Agarwal S, Sikarwar S (2012b) Photo-catalytic degradation of toxic dye amaranth on TiO2/UV in aqueous suspensions. Mater Sci Eng, C 32(1):12–17
Gupta VK, Kumar R, Nayak A, Saleh TA, Barakat MA (2013) Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: a review. Adv Colloid Interface 193–194:24–34
Haman C, Dauchy X, Rosin C, Munoz J (2015) Occurrence, fate and behavior of parabens in aquatic environments: a review. Water Res 68:1–11
Hosseini M, Memari Z, Ganjali MR, Khoobi M, Faridbod F, Shafiee A, Norouzi P, Shamsipur M, Hajinezhad A (2014) A novel mercury-sensitive fluorescent nano-chemosensor using new functionalized magnetic core-shell Fe3O4 @SiO2 nanoparticles. Int J Environ Res 8(4):861–870
Huang Y, Cui C, Zhang D, Li L, Pan D (2015) Heterogeneous catalytic ozonation of dibutyl phthalate in aqueous solution in the presence of iron-loaded activated carbon. Chemosphere 119:295–301
Ichikawa S, Mahardiani L, Kamiya Y (2014) Catalytic oxidation of ammonium ion in water with ozone over metal oxide catalysts. Catal Today 232:192–197
Jonkers N, Sousa A, Galante-Oliveira S, Barroso CM, Kohler HE, Giger W (2010) Occurrence and sources of selected phenolic endocrine disruptors in Ria de Aveiro, Portugal. Environ Sci Pollut Res 17(4):834–843
Kasprzyk-Hordern B (2003) Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl Catal B Environ 46(4):639–669
Lee E, Lee H, Kim YK, Sohn K, Lee K (2011) Hydrogen peroxide interference in chemical oxygen demand during ozone based advanced oxidation of anaerobically digested livestock wastewater. Int J Environ Sci Technol 8(2):288–381
Li J, Na H, Zeng X, Zhu T, Liu Z (2014) In situ DRIFTS investigation for the oxidation of toluene by ozone over Mn/HZSM-5, Ag/HZSM-5 and Mn–Ag/HZSM-5 catalysts. Appl Surf Sci 311:690–696
Mittal A, Kaur D, Malviya A, Mittal J, Gupta VK (2009) Adsorption studies on the removal of coloring agent phenol red from wastewater using waste materials as adsorbents. J Colloid Interface Sci 337(2):345–354
Moussavi G, Aghapour AA, Yaghmaeian K (2014) The degradation and mineralization of catechol using ozonation catalyzed with MgO/GAC composite in a fluidized bed reactor. Chem Eng J 249:302–310
Parfiti GD (1976) Surface chemistry of oxides. Pure Appl Chem 48:415–418
Salado J, Insausti M, Gil De Muro I, Lezama L, Rojo T (2008) Synthesis and magnetic properties of monodisperse Fe3O4 nanoparticles with controlled sizes. J Non-Cryst Solids 354(47–51):5207–5209
Saleh TA, Gupta VK (2011) Functionalization of tungsten oxide into MWCNT and its application for sunlight-induced degradation of rhodamine B. J Colloid Interface Sci 362(2):337–344
Saleh TA, Gupta VK (2012a) Photo-catalyzed degradation of hazardous dye methyl orange by use of a composite catalyst consisting of multi-walled carbon nanotubes and titanium dioxide. J Colloid Interface Sci 371(1):101–106
Saleh TA, Gupta VK (2012b) Column with CNT/magnesium oxide composite for lead (II) removal from water. Environ Sci Pollut Res 19(4):1224–1228
Saleh TA, Gupta VK (2012c) Synthesis and characterization of alumina nano-particles polyamide membrane with enhanced flux rejection performance. Sep Purif Technol 89:245–251
Saleh TA, Gupta VK (2014) Processing methods, characteristics and adsorption behavior of tire derived carbons: a review. Adv Colloid Interface 211:93–101
Saleh TA, Agarwal S, Gupta VK (2011) Synthesis of MWCNT/MnO2 and their application for simultaneous oxidation of arsenite and sorption of arsenate. Appl Catal B Environ 106(1–2):46–53
Tanaka KA, Abe K (1997) Fe3+ and UV enhanced ozonation of chlorophenolic compounds in aqueous medium. Chemosphere 12(15):2837–2847
Triki M, Ksibi Z, Ghorbel A, Medina F (2011) Preparation and characterization of CeO2–Al2O3 aerogels supported ruthenium for catalytic wet air oxidation of p-hydroxybenzoic acid. J Sol–Gel Sci Technol 59(1):1–6
Turkay O, Inan H, Dimoglo A (2014) Experimental and theoretical investigations of CuO-catalyzed ozonation of humic acid. Sep Purif Technol 134:110–116
Usharani K, Muthukumar M, Kadirvelu K (2012) Effect of pH on the degradation of aqueous organophosphate (methylparathion) in wastewater by ozonation. Int J Environ Res 6(2):557–564
Valdés H, Zaror CA (2006) Heterogeneous and homogeneous catalytic ozonation of benzothiazole promoted by activated carbon: kinetic approach. Chemosphere 65(7):1131–1136
Wang Z (2015) Efficient adsorption of dibutyl phthalate from aqueous solution by activated carbon developed from phoenix leaves. Int J Environ Sci Technol 12(6):1923–1932
Wang JL, Xu LJ (2012) Advanced oxidation processes for wastewater treatment: formation of hydroxyl radical and application. Crit Rev Environ Sci Tecnol 42(3):251–325
Xing L, Xie Y, Cao H, Minakata D, Zhang Y, Crittenden JC (2014) Activated carbon-enhanced ozonation of oxalate attributed to HO oxidation in bulk solution and surface oxidation: effects of the type and number of basic sites. Chem Eng J 245:71–79
Xu L, Wang J (2012a) Fenton-like degradation of 2, 4-dichlorophenol using Fe3O4 magnetic nanoparticles. Appl Catal B 123–124(18):117–126
Xu L, Wang J (2012b) Magnetic nanoscaled Fe3O4/CeO2 composite as an efficient Fenton-like heterogeneous catalyst for degradation of 4-chlorophenol. Environ Sci Technol 46:10145–10153
Yang L, Hu C, Nie Y, Qu J (2010) Surface acidity and reactivity of β-FeOOH/Al2O3 for pharmaceuticals degradation with ozone: in situ ATR-FTIR studies. Appl Catal B Environ 97(3–4):340–346
Yang Y, Cao H, Peng P, Bo H (2014) Degradation and transformation of atrazine under catalyzed ozonation process with TiO2 as catalyst. J Hazard Mater 279:444–451
Zhang S, Wang D, Quan X, Zhou L, Zhang X (2013) Multi-walled carbon nanotubes immobilized on zero-valent iron plates (Fe0-CNTs) for catalytic ozonation of methylene blue as model compound in a bubbling reactor. Sep Purif Technol 116:351–359
Acknowledgments
The research was supported by the Program for Changjiang Scholars and Innovative Research Team in University (IRT-13026). The authors would also like to thank the financial support provided by the National Natural Science Foundation of China (Grant No. 51338005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bai, Z.Y., Yang, Q. & Wang, J.L. Fe3O4/multi-walled carbon nanotubes as an efficient catalyst for catalytic ozonation of p-hydroxybenzoic acid. Int. J. Environ. Sci. Technol. 13, 483–492 (2016). https://doi.org/10.1007/s13762-015-0881-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-015-0881-3