Abstract
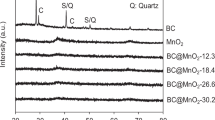
Biochar (BC) derived from pyrolysis of plant biomass can be used as an inexpensive adsorbent for removal of aqueous heavy metals. In the present study, amorphous hydrous manganese oxide was loaded onto BC to fabricate a novel manganese-oxide/biochar (Mn/BC) composite for further enhancing lead(II) removal by sorption from water. Mn/BC was characterized by X-ray diffraction, scanning electron microscope, and specific surface area analysis. After impregnation with 3.65 % Mn, BC raised the removal efficiency of lead(II) from 6.4 to 98.9 % at pH 5.00. This improvement is attributed to the increase in surface hydroxyls and the decrease in pHPZC (pH at the point of zero charge) of carbon. The maximum monolayer adsorption of lead(II) on Mn/BC at 298 K was five times that on BC. The smaller pseudo-second-order rate constant of Mn/BC compared with that of BC indicated that the sorption rate of Mn/BC is faster than that of BC. The calculated thermodynamic parameters implied that the sorption of lead(II) is a spontaneous and endothermic process. The results suggest that Mn/BC composite is a promising adsorbent for the efficient removal of lead(II) from water.





Similar content being viewed by others
References
Aksu Z (2002) Determination of the equilibrium, kinetic and thermodynamic parameters of the batch biosorption of lead (II) ions onto Chlorella vulgaris. Process Biochem 38:89–99
Cao XD, Ma LN, Gao B, Harris W (2009) Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ Sci Technol 43:3285–3291
Chen ZM, Chen BL, Chiou CT (2012) Fast and slow rates of naphthalene sorption to biochars produced at different temperatures. Environ Sci Technol 46:11104–11111
Chun Y, Sheng G, Chiou CT, Xing BS (2004) Compositions and sorptive properties of crop residue-derived chars. Environ Sci Technol 38:4649–4655
Considine R, Denoyel R, Pendleton P, Schumann R, Wong SH (2001) The influence of surface chemistry on activated carbon adsorption of 2-methylisoborneol from aqueous solution. Colloid Surf A 179:271–280
Eren E, Afsin B, Onal Y (2009) Removal of lead ions by acid activated and manganese oxide-coated bentonite. J Hazard Mater 161:677–685
Fan HJ, Anderson PR (2005) Copper and cadmium removal by Mn oxide-coated granular activated carbon. Sep Purif Technol 45:61–67
Fan X, Parker DJ, Smith MD (2003) Adsorption kinetics of fluoride on low cost materials. Water Res 37:4929–4937
Fraser B (2010) High-tech charcoal fights climate change. Environ Sci Technol 44:548–549
Guo HM, Li Y, Zhao K, Ren Y, Wei C (2011) Removal of arsenite from water by synthetic siderite: behaviors and mechanisms. J Hazard Mater 186:1847–1854
Han RP, Zou WH, Li HK, Li YH, Shi J (2006a) Copper(II) and lead(II) removal from aqueous solution in fixed-bed columns by manganese oxide coated zeolite. J Hazard Mater 137:934–942
Han RP, Zou WH, Zhang ZP, Shi J, Yang JJ (2006b) Removal of copper(II) and lead(II) from aqueous solution by manganese oxide coated sand I. characterization and kinetic study. J Hazard Mater 137:384–395
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Keiluweit M, Nico PS, Johnson MG, Kleber M (2010) Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ Sci Technol 44:1247–1253
Kong HL, He J, Gao YZ, Wu HF, Zhu XZ (2011) Cosorption of phenanthrene and mercury (ii) from aqueous solution by soybean stalk-based biochar. J Agric Food Chem 59:12116–12123
Lenoble V, Laclautre C, Serpaud B, Deluchat V, Bollinger JC (2004) As(V) retention and As(III) simultaneous oxidation and removal on a MnO2-loaded polystyrene resin. Sci Total Environ 326:197–207
Lin YR, Teng HS (2002) Mesoporous carbons from waste tire char and their application in wastewater discoloration. Micropor Mesopor Mater 54:167–174
Liu RP, Liu HJ, Qiang ZM, Qu JH, Li GB, Wang DS (2009) Effects of calcium ions on surface characteristics and adsorptive properties of hydrous manganese dioxide. J Colloid Interf Sci 331:275–280
Lv L, He J, Wei M, Evans DG, Zhou ZL (2007) Treatment of high fluoride concentration water by MgAl-CO3 layered double hydroxides: kinetic and equilibrium studies. Water Res 41:1534–1542
Maliyekkal MS, Sharma AK, Philip L (2006) Manganese-oxide-coated alumina: a promising sorbent for defluoridation of water. Water Res 40:3497–3506
Pretorius PJ, Lindner PW (2001) The adsorption characteristics of δ-manganese dioxide: a collection of diffuse double layer constants for the adsorption of H+, Cu2+, Ni2+, Zn2+, Cd2+ and Pb2+. Appl Geochem 16:1067–1082
Qiu YP, Cheng HY, Xu C, Sheng GD (2008) Surface characteristics of crop-residue-derived black carbon and lead(II) adsorption. Water Res 42:567–574
Qiu H, Pan BC, Zhang QJ, Zhang WM, Zhang QX (2009a) Critical review in adsorption kinetic models. J Zhejiang Univ Sci A 10:716–724
Qiu YP, Zheng ZZ, Zhou ZL, Sheng GD (2009b) Effectiveness and mechanisms of dye adsorption on a straw-based biochar. Bioresour Technol 100:5348–5351
Reddad Z, Gerente C, Andres Y, Cloirec PL (2002) Adsorption of several metal ions onto a low-cost biosorbent: kinetic and equilibrium studies. Environ Sci Technol 36:2067–2073
Sharaf G, Hassan H (2013) Removal of copper ions from aqueous solution using silica derived from rice straw: comparison with activated charcoal. Int J Environ Sci Technol. doi:10.1007/s13762-013-0343-8
Shuang CD, Li PH, Li AM, Zhou Q, Zhang MC, Zhou Y (2012) Quaternized magnetic microspheres for the efficient removal of reactive dyes. Water Res 46:4417–4426
Şölener M, Tunali S, Özcan AS, Özcan A, Gedikbey T (2008) Adsorption characteristics of lead(II) ions onto the clay/poly(methoxyethyl)acrylamide (PMEA) composite from aqueous solutions. Desalination 223:308–322
Su Q, Pan BC, Pan BJ, Zhang QR, Zhang WM, LV L, Wang XS, Wu J, Zhang QX (2009) Fabrication of polymer-supported nanosized hydrous manganese dioxide (HMO) for enhanced lead removal from waters. Sci Total Environ 407:5471–5477
Thipnakarin B, Axe L, Xu Y, Tyson TA (2006) Nickel and lead sequestration in manganese oxide-coated montmorillonite. J Colloid Interf Sci 303:87–98
Uchimiya M, Chang S, Klasson KT (2011) Screening biochars for heavy metal retention in soil: role of oxygen functional groups. J Hazard Mater 190:432–441
Vasudevan D, Dorley PJ, Zhuang X (2001) Adsorption of hydroxy pyridines and quinolines at the metal oxide-water interface: role of tautomeric equilibrium. Environ Sci Technol 35:2006–2013
Wang SG, Gong WX, Liu XW, Yao YW, Gao BY, Yue QY (2007) Synthesis and characterization of manganese dioxide spontaneously coated on carbon nanotubes. Sep Purif Technol 58:17–23
Wang Y, Wang L, Fang GD, Herath HMSK, Wang YJ, Cang L, Xie ZB, Zhou DM (2013) Enhanced PCBs sorption on biochars as affected by environmental factors: humic acid and metal cations. Environ Pollut 72:86–93
Wu XQ, Xiao BD, Li RH, Wang CB, Huang JT, Wang Z (2011) Mechanisms and factors affecting sorption of microcystins onto natural sediments. Environ Sci Technol 45:2641–2647
Zhang J, Shi QQ, Zhang CL, Xu JT, Zhai B, Zhang B (2008) Adsorption of neutral red onto Mn-impregnated activated carbons prepared from Typha orientalis. Bioresour Technol 99:8974–8980
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 21177113).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, M.C., Sheng, G.D. & Qiu, Y.P. A novel manganese-oxide/biochar composite for efficient removal of lead(II) from aqueous solutions. Int. J. Environ. Sci. Technol. 12, 1719–1726 (2015). https://doi.org/10.1007/s13762-014-0538-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-014-0538-7