Abstract
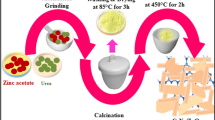
In this paper, four TiO2/β-FeOOH photocatalysts were synthesized by a simple deposition–precipitation method and characterized by X-ray diffraction, fourier transform infrared, scanning electron microscope and transmission electron microscope. The characterization showed the presence of nano-sized β-FeOOH particles on the TiO2 support. The photocatalytic efficiency of the catalysts was examined on the Cr(VI) reduction under ultraviolet irradiation in aqueous suspension. The photocatalyst denoted as 25TiO2/β-FeOOH appeared to be most efficient, which is due to effectively inhibiting the recombination of photoinduced electrons and holes. A 24 factorial design methodology was employed to evaluate the statistically important operating conditions (pH of the solution, loading of catalyst, Cr(VI) concentration and reaction time) and their interactions on the photocatalytic reduction efficiency of Cr(VI) over 25TiO2/β-FeOOH.






Similar content being viewed by others
References
Banić N, Abramović B, Krstić J, Šojić D, Lončarević D, Cherkezova-Zheleva Z, Guzsvány V (2011) Photodegradation of thiacloprid using Fe/TiO2 as a heterogeneous photo-Fenton catalyst. Appl Catal B 107:363–371
Barrera-Díaz CE, Lugo-Lugo V, Bilyeu B (2012) A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J Hazard Mater 223–224:1–12
Benz M, van der Kraan AM, Prins R (1998) Reduction of aromatic nitrocompounds with hydrazine hydrate in the presence of an iron oxide hydroxide catalyst—II. Activity, X-ray diffraction and Mossbauer study of the iron oxide hydroxide catalyst. Appl Catal A 172:149–157
Box GEP, Hunter WG, Hunter JS (1978) Statistics for experimenters. Wiley, New York
Cespón-Romero RM, Yebra-Biurrun MC, Bermejo-Barrera MP (1996) Preconcentration and speciation of chromium by the determination of total chromium and chromium(III) in natural waters by flame atomic absorption spectrometry with a chelating ion-exchange flow injection system. Anal Chim Acta 327:37–45
Cheng ZW, Zhao DN (2011) Effects of experimental conditions on one-dimensional single-crystal nanostructure of β-FeOOH. Mater Chem Phys 127:220–226
Eary LE, Rai D (1988) Chromate removal from aqueous wastes by reduction with ferrous ion. Environ Sci Technol 22:972–977
Eary LE, Rai D (1991) Chromate reduction by subsurface soils under acidic conditions. Soil Sci Soc Am J 55:676–683
Fresno F, Herna’ndez-Alonso MD, David T, Coronado JM, Soria J (2008) Photocatalytic degradation of toluene over doped and coupled (Ti, M)O2 (M=Sn or Zr) nanocrystalline oxides: influence of the heteroatom distribution on deactivation. Appl Catal B 84:598–606
Jiang F, Zheng Z, Xu ZY, Zheng SR, Guo ZB, Chen LQ (2006) Aqueous Cr(VI) photo-reduction catalyzed by TiO2 and sulfated TiO2. J Hazard Mater B134:94–103
Konlen’ko YV, Churagulov BR, Kunst M, Mazerolles L, Colbeau-Justin C (2004) Photocatalytic properties of titania powders prepared by hydrothermal method. Appl Catal B 54:51–58
Ku Y, Jung IL (2001) Photocatalytic reduction of Cr(VI) in aqueous solutions by UV irradiation with the presence of titanium dioxide. Water Res 35:135–142
Li JX, Xu JH, Dai WL, Li HX, Fan KN (2009) Direct hydro-alcohol thermal synthesis of special core–shell structured Fe-doped titania microspheres with extended visible light response and enhanced photoactivity. Appl Catal B 85:162–170
Liang X, Wang X, Zhuang J, Chen YT, Wang DS, Li YD (2006) Synthesis of nearly monodisperse iron oxide and oxyhydroxide nanocrystals. Adv Funct Mater 16:1805–1813
Liu XM, Fu SY, Xiao HM, Huang CJ (2005) Preparation and characterization of shuttle-like α-Fe2O3 nanoparticles by supermolecular template. J Solid State Chem 178:2798–2803
Liu LF, Chen F, Yang FL, Chen YS, Crittenden J (2012) Photocatalytic degradation of 2, 4-dichlorophenol using nanoscale Fe/TiO2. Chem Eng J 181:189–195
Myers RH, Montgomery DC (2002) Response surface methodology: process and product optimization using designed experiments. Wiley, New Jersey
Nriagu J, Nieboer E (1988) Chromium in the natural and human environments. Wiley, New York
Olmez T (2009) The optimization of Cr(VI) reduction and removal by electrocoagulation using response surface methodology. J Hazard Mater 162:1371–1378
Pal B, Sharon M, Nogami G (1999) Preparation and characterization of TiO2/Fe2O3 binary mixed oxides and its photocatalytic properties. Mater Chem Phys 59:254–261
Parida KM, Sahu N (2008) Visible light induced photocatalytic activity of rare earth titania nanocomposites. J Mol Catal A: Chem 287:151–158
Qiu RL, Zhang DD, Diao ZH, Huang XF, He C, Morel JL, Xiong Y (2012) Visible light induced photocatalytic reduction of Cr(VI) over polymer-sensitized TiO2 and its synergism with phenol oxidation. Water Res 46:2299–2306
Rengaraj S, Venkataraj S, Yeon JW, Kim YH, Li XZ, Pang GKH (2007) Preparation, characterization and application of Nd-TiO2 photocatalyst for the reduction of Cr(VI) under UV light illumination. Appl Catal B 77:157–165
Ristic M, Music S, Orehovec Z (2005) Thermal decomposition of synthetic ammonium jarosite. J Mol Struct 744:295–300
Singh KP, Singh AK, Gupta S, Sinha S (2011) Optimization of Cr(VI) reduction by zero-valent bimetallic nanoparticles using the response surface modeling approach. Desalin 270:275–284
Štajdohar J, Ristić M, Musić S (2012) Development of porous α-Fe2O3 microstructure by forced hydrolysis of FeCl3 solutions in the presence of AOT. J Alloys Compd 532:41–48
Sugimoto T, Muramatsu A (1996) Formation mechanism of monodispersed α-Fe2O3 particles in dilute FeCl3 solutions. J Colloid Interface Sci 184:626–638
Sun Q, Leng WH, Li Z, Xu YM (2012) Effect of surface Fe2O3 clusters on the photocatalytic activity of TiO2 for phenol degradation in water. J Hazard Mater 229–230:224–232
Tong GX, Guan JG, Zhang QJ (2011) Goethite hierarchical nanostructures: glucose-assisted synthesis, chemical conversion into hematite with excellent photocatalytic properties. Mater Chem Phys 127:371–378
Wang ZH, Jiang TS, Du YM, Chen KM, Yin HB (2006) Synthesis of mesoporous titania and the photocatalytic activity for decomposition of methyl orange. Mater Lett 60:2493–2496
Xu ZH, Lǚ B, Wu JY, Zhou LX, Lan YQ (2013) Reduction of Cr(VI) facilitated by biogenetic jarosite and analysis of its influencing factors with response surface methodology. Mater Sci Eng C 33:3723–3729
Yoon J, Shim E, Bae S, Joo H (2009) Application of immobilized nanotubular TiO2 electrode for photocatalytic hydrogen evolution: reduction of hexavalent chromium (Cr(VI)) in water. J Hazard Mater 161:1069–1074
Yu L, Peng XJ, Ni F, Li J, Wang DS, Luan ZK (2013) Arsenite removal from aqueous solutions by γ-Fe2O3–TiO2 magnetic nanoparticles through simultaneous photocatalytic oxidation and adsorption. J Hazard Mater 246–247:10–17
Yuan ZY, Ren TZ, Su BL (2004) Surfactant mediated nanoparticle assembly of catalytic mesoporous crystalline iron oxide materials. Catal Today 93–95:743–750
Yurik TK, Pikaev AK (1999) Radiolysis of weakly acidic and neutral aqueous solutions of hexavalent chromium ions. High Energy Chem 33:208–212
Zhang CY, Wang JL, Zhou HF, Fu DG, Gu ZZ (2010) Anodic treatment of acrylic fiber manufacturing wastewater with boron-doped diamond electrode: a statistical approach. Chem Eng J 161:93–98
Zhao YP, Hu JY, Chen HB (2010) Elimination of estrogen and its estrogenicity by heterogeneous photo-Fenton catalyst beta-FeOOH/resin. J Photochem Photobiol A 212:94–100
Acknowledgments
This work was supported by the National Natural Science Foundation of China (40930738, 21077053). The authors would like to thank Mr. Jianjian Wang for his excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, M., Xu, Z., Liang, J. et al. Potential application of novel TiO2/β-FeOOH composites for photocatalytic reduction of Cr(VI) with an analysis of statistical approach. Int. J. Environ. Sci. Technol. 12, 1669–1676 (2015). https://doi.org/10.1007/s13762-014-0533-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-014-0533-z