Abstract
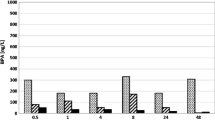
The aim of this research was to assess the efficiency of Fenton’s oxidation for degradation of endocrine disruptor bisphenol A (BPA) with emphasis on extent of accompanying adsorption. Adsorption on the waste sludge resulting from the Fenton’s oxidation could represent a significant impact on the final removal efficiency of BPA. Fenton’s oxidation was accomplished at two concentrations of BPA (0.228 and 22.8 mg L−1); both at the selected molar ratio of reagents Fe2+:H2O2 (1:10), as a function of reaction time. The kinetics of adsorption of BPA on waste sludge was determined for the same two concentrations of BPA at two concentrations of waste sludge (0.1 and 6.0 g L−1). In addition to changing concentrations of BPA and sludge, the adsorption process was also influenced by parameters such as temperature, pH and contact time. Adsorption isotherms were determined. Oxidation and adsorption were monitored by gas chromatography combined with mass spectrum. It has been confirmed that BPA is not completely oxidized in Fenton’s oxidation, because it is adsorbed to formed waste ferric sludge and thus necessary precautions for sludge deposition must be observed.





Similar content being viewed by others
References
Abo-Farha A (2010) Comparative study of oxidation of some azo dyes by different advanced oxidation processes: Fenton, Fenton-like, photo-Fenton. J Am Sci 6:128–142
Andreozzi R, Caprio V, Insola A, Marotta R (1999) Advanced oxidation processes (AOP) for water purification and recovery. Catal Today 53:51–59
Bansal RC, Goyal M (2005) Activated carbon adsorption. CRC Press/Taylor & Francis Group, Boca Raton, pp 145–159
Brondi SHG, Lancas FM (2005) Optimization of a methodology for the determination of organochlorine pesticides in surface water by SPME-GC/MS. J Environ Sci Health A 40:513–523
Citulski JA, Farahbakhsh K (2010) Fate of endocrine-active compounds during municipal biosolids treatment: a review. Environ Sci Technol 44:8367–8376
Dong Y, Wu D, Chen X, Lin Y (2010) Adsorption of bisphenol A from water by surfactant-modified zeolite. J Colloid Interface Sci 348:585–590
Eisert R, Levsen K (1996) Solid-phase microextraction coupled to gas chromatography: a new method for the analysis of organics in water. J Chromatogr 733:143–157
Farre M, Perez S, Katiani L, Barcelo D (2008) Fate and toxicity of emerging pollutants, their metabolites and transformation products in the aquatic environment. Trends Anal Chem 27:991–1007
Fei Y, Li X-D, Li X-Y (2011) Organic diagenesis in sediment and its impact on the adsorption of bisphenol A and nonylphenol onto marine sediment. Mar Pollut Bull 63:578–582
Fujimoto T, Kubo K, Aou S (2007) Environmental impacts on brain functions: low dose effects of bisphenol A during perinatal critical period. Int Congr Ser 1301:226–229
Ho YS, McKay G (1998) A comparison of chemisorptions kinetic models applied to pollutant removal on various sorbents. Process Saf Environ 76(4):332–340
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Kang W, Hwang KY (2000) Effects of reaction conditions on the oxidation efficiency in the Fenton process. Water Res 34:2786–2790
Kang JH, Kondo F, Katayama Y (2006) Human exposure to bisphenol A. Toxicology 226:79–89
Katsumata H, Kawebe S, Kaneco S, Suzuki T, Ohta K (2004) Degradation of bisphenol A in water by the photo-Fenton reaction. J Photochem Photobiol A Chem 162:297–305
Kavitha V, Palanivelu K (2004) The role of ferrous ion in Fenton and photo-Fenton for the degradation of phenol. Chemosphere 55:1235–1243
Kazner C, Lehnberg K, Kovalova L, Wintgens T, Melin T, Hollender J, Dott W (2008) Removal of endocrine and cytostatics from effluent by nanofiltration in combination with adsorption on powdered activated carbon. Water Sci Technol 8:1699–1706
Khanal SK, Xie B, Thompson ML, Sung S, Ong S-K, van Leeuvent J (2006) Fate, transport and biodegradation of natural estrogens in the environment and engineered systems. Environ Sci Technol 40(21):6537–6546
Kolpin DW, Furlong ET, Meyer MT, Thurman M, Zaugg SD, Barber LB, Buxton HT (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: a national reconnaissance. Environ Sci Technol 36:1202–1211
Lagergren S (1898) Zur theorie der sogenannten adsorption gelöster stoffe, Kungliga Svenska Vetenskapsakademiens. Handlingar 24:1–39 (in Swedish)
Ma X-J, Xia H-L (2009) Treatment of water-based printing ink wastewater by Fenton process combined with coagulation. J Hazard Mater 162:386–390
Mlynarcikova A, Kolena J, Fickova M, Scsukova S (2005) Alterations in steroid hormone production by porcine ovarian granulosa cells caused by bisphenol A and bisphenol A dimethacrylate. Mol Cell 244:57–62
Movahedyan H, Seid Mohammadi AM, Assadi A (2009) Comparison of different advanced oxidation processes degrading p-chlorophenol in aqueous solution. Iran J Environ Health Sci Eng 6:153–160
Neyens E, Baeyens J (2003) A review of classic Fenton’s peroxidation as an advanced oxidation technique. J Hazard Mater B98:33–50
Prousek J (1996) Advanced oxidation processes for water treatment. Chemical process. Chem Listy 90:229–237
Qiang Z, Chang J-H, Huang C-P (2003) Electrochemical regeneration of Fe2+ in Fenton oxidation processes. Water Res 37:1308–1319
Qiu H, Lv L, Pan B, Zhang Q, Zhang W, Zhang Q (2009) Critical review in adsorption kinetic models. J Zhejiang Univ Sci A 10(5):716–724
Simon P (2004) Advanced oxidation processes for water and wastewater treatment. IWA Publishing, London
Staples CA, Dorn PB, Klecka GM, O’Block ST, Harris LR (1998) A review of the environmental fate, effects and exposures of bisphenol A. Chemosphere 36(10):2149–2173
Tan IAW, Ahmad AL, Hameed BH (2008) Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: equilibrium, kinetic and thermodynamic studies. J Hazard Mater 154(1–3):337–346
Ternes T, Joss A (2006) Human pharmaceuticals, hormones and flagrances: the challenge of micropollutants in urban water management. IWA Publishing, London, pp 45–52
Tsai W, Ali C, Su T (2006) Adsorption of bisphenol A from aqueous solution onto minerals and carbon adsorbents. J Hazard Mater B134:169–175
Yamamoto T, Yasuhara A, Shiraishi H, Nakasugi O (2001) Bisphenol A in hazardous waste landfill leachates. Chemosphere 42:415–418
Zeng G, Zhang C, Huang G, Yu J, Wang Q, Li J, Xi B, Liu H (2006) Adsorption behaviour of bisphenol A on sediments in Xiangjiang River, Central-south China. Chemosphere 65:1490–1499
Zoeller RT, Bansal R, Parris C (2005) Bisphenol-A an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro increases serum thyroxine and alters RC3/neurogranin expression in the developing rat brain. Endocrinology 146:607–612
Acknowledgments
Authors are thankful to Mr. Matjaž Pevec and Mrs. Jana Nakrst for their excellent technical assistance. This work was supported by Slovenian Research Agency (ARRS) as a part of bilateral USA—Slovenia agreement BI/US/11-12-037 (2011–2012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Žgajnar Gotvajn, A., Bistan, M., Tišler, T. et al. The relevance of bisphenol A adsorption during Fenton’s oxidation. Int. J. Environ. Sci. Technol. 10, 1141–1148 (2013). https://doi.org/10.1007/s13762-012-0153-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-012-0153-4