Abstract
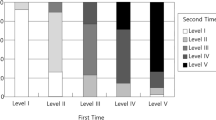
Gross motor dysfunction is considered as the most challenging problem in cerebral palsy (CP). It is proven that improvement of gross motor function could reduce CP-related disabilities and provide better quality of life in this group of patients. Therefore, the aim of this trial is to evaluate the effectiveness of cerebrolysin (CBL) on gross motor function of children with CP who are undergoing treatment. In this clinical trial study, paediatric patients aged 18–75 months with spastic diplegic or quadriplegic cerebral palsy, who were under rehabilitation therapy, were selected and randomly allocated in control and CBL groups. Patients in CBL group underwent treatment with standard rehabilitation therapy plus CBL. The latter was administrated intramuscularly as a single daily dose of 0.1 cc/kg for 10 days and then continued weekly for 4 months. Gross motor function of participants in the two studied groups, before and after trial, was evaluated and compared using the validated Persian version of gross motor function classification system-expanded and revised (GMFCS-E&R). During this trial, 108 patients with CP were evaluated for eligibility. From these, 50 patients were enrolled and randomly allocated in the CBL and control groups. Four months after trial, the mean level of GMFCS decreased significantly in the two groups (P < 0.05). However, it was significantly lower in the CBL group than in the control group (2.1 vs. 3.16, P < 0.05). The results of this trial indicated that CBL could improve gross motor function in patients with CP. This finding is consistent with neurotrophic and neuroprotective effects of CBL, which have been reported in various clinical trials in other neurological disorders. Further studies are recommended to establish the value of continued neuroprotection and to determine the pharmacokinetics/dynamics of CBL in this group of patients.


Similar content being viewed by others
References
Jones MW, Morgan E, Shelton JE, Thorogood C (2007) Cerebral palsy: introduction and diagnosis (part I). J Pediatr Health Care Off Publ Natl Assoc Pediatr Nurse Assoc Pract 21(3):146–152
Shevell MI, Bodensteiner JB (2004) Cerebral palsy: defining the problem. Sem Pediatr Neurol 11(1):2–4
Bax M, Goldstein M, Rosenbaum P, Leviton A, Paneth N, Dan B et al (2005) Proposed definition and classification of cerebral palsy. Dev Med Child Neurol 47(8):571–576
Odding E, Roebroeck ME, Stam HJ (2006) The epidemiology of cerebral palsy: incidence, impairments and risk factors. Disabil Rehabil 28(4):183–191
Miller F (2005) Cerebral palsy. Springer Science-Business Media, New York, pp 523–666
labaf S, Shamsoddini A, Hollisaz MT, Sobhani V, Shakibaee A (2015) Effects of neurodevelopmental therapy on gross motor function in children with cerebral palsy. Iran J Child Neurol 9(1):36–41
Shamsoddini A, Amirsalari S, Hollisaz MT, Rahimnia A, Khatibi-Aghda A (2014) Management of spasticity in children with cerebral palsy. Iran. J Pediatr 24(4):345–351
Wittenberg GF (2010) Experience, cortical remapping, and recovery in brain disease. Neurobiol Dis 37(2):252–258
Carroll JE, Mays RW (2011) Update on stem cell therapy for cerebral palsy. Expert Opin Biol Ther 11(4):463–471
Balakrishnan B, Nance E, Johnston MV, Kannan R, Kannan S (2013) Nanomedicine in cerebral palsy. Int J Nanomed 8:4183–4195
Aisen ML, Kerkovich D, Mast J, Mulroy S, Wren TA, Kay RM, Rethlefsen SA (2011) Cerebral palsy: clinical care and neurological rehabilitation. Lancet Neurol 10(9):844–852
Alvarez XA, Cacabelos R, Laredo M, Couceiro V, Sampedro C, Varela M et al (2006) A 24-week, double-blind, placebo-controlled study of three dosages of cerebrolysin in patients with mild to moderate Alzheimer’s disease. Eur J Neurol Off J Eur Fed Neurol Soc 13(1):43–54
Rockenstein E, Adame A, Mante M, Larrea G, Crews L, Windisch M et al (2005) Amelioration of the cerebrovascular amyloidosis in a transgenic model of Alzheimer’s disease with the neurotrophic compound cerebrolysin. J Neural Transm 112(2):269–282
Amiri-Nikpour MR, Nazarbaghi S, Ahmadi-Salmasi B, Mokari T, Tahamtan U, Rezaei Y (2014) Cerebrolysin effects on neurological outcomes and cerebral blood flow in acute ischemic stroke. Neuropsychiatr Dis Treat 10:2299–2306
Zhang Y, Chopp M, Meng Y, Zhang ZG, Doppler E, Winter S et al (2015) Cerebrolysin improves cognitive performance in rats after mild traumatic brain injury. J Neurosurg 122(4):1–13
Masliah E, Diez-Tejedor E (2012) The pharmacology of neurotrophic treatment with cerebrolysin: brain protection and repair to counteract pathologies of acute and chronic neurological disorders. Drugs Today (Barc) 48(Suppl A):3–24
Chen N, Yang M, Guo J, Zhou M, Zhu C, He L (2013) Cerebrolysin for vascular dementia. Cochrane Database Syst Rev 1:CD008900
Alvarez XA, Cacabelos R, Sampedro C, Aleixandre M, Linares C, Granizo E et al (2011) Efficacy and safety of Cerebrolysin in moderate to moderately severe Alzheimer’s disease: results of a randomized, double-blind, controlled trial investigating three dosages of cerebrolysin. Eur J Neurol Off J Eur Fed Neurol Soc 18(1):59–68
Heiss WD, Brainin M, Bornstein NM, Tuomilehto J, Hong Z (2012) Cerebrolysin in patients with acute ischemic stroke in Asia: results of a double-blind, placebo-controlled randomized trial. Stroke J Cereb Circ 43(3):630–636
Thome J, Doppler E (2012) Safety profile of cerebrolysin: clinical experience from dementia and stroke trials. Drugs Today (Barc) 48(Suppl A):63–69
Dehghan L, Dalvand H, Abdolvahab M, Bagheri H, Faghih zade S (2011) Inter rater reliability of Persian version Gross Motor Function Classification System Expanded & Revised in patients with cerebral palsy. Bimonthly Official Publication Medical Daneshvar 18(91):37–44
Sharma HS, Muresanu DF, Patnaik R, Stan AD, Vacaras V, Perju-Dumbrav L et al (2011) Superior neuroprotective effects of cerebrolysin in heat stroke following chronic intoxication of Cu or Ag engineered nanoparticles. A comparative study with other neuroprotective agents using biochemical and morphological approaches in the rat. J Nanosci Nanotechnol 11(9):7549–7569
Young W (2009) Cerebrolysin Review. Available from https://wiseyoung.wordpress.com/2009/02/10/271/. Accessed 22 Dec 2016
Lukhanina EP, Karaban IN, Burenok Iu A, Mel’nik NA, Berezetskaia NM (2004) Zhurnal nevrologii i psikhiatrii imeni SS Korsakova/Ministerstvo zdravookhraneniia i meditsinskoi promyshlennosti Rossiiskoi Federatsii, Vserossiiskoe obshchestvo nevrologov [Effect of cerebrolysin on the electroencephalographic indices of brain activity in Parkinson’s disease] [i] Vserossiiskoe obshchestvo psikhiat 104(7):54–60
Doppler E, Rockenstein E, Ubhi K, Inglis C, Mante M, Adame A et al (2008) Neurotrophic effects of cerebrolysin in the Mecp2 (308/Y) transgenic model of Rett syndrome. Acta Neuropathol 116(4):425–437
Hutter-Paier B, Steiner E, Windisch M (1998) Cerebrolysin protects isolated cortical neurons from neurodegeneration after brief histotoxic hypoxia. J Neural Transm Suppl 53:351–361
Hutter-Paier B, Fruhwirth M, Grygar E, Windisch M (1996) Cerebrolysin protects neurons from ischemia-induced loss of microtubule—associated protein 2. J Neural Transm Suppl 47:276
Ruther E, Ritter R, Apecechea M, Freytag S, Windisch M (1994) Efficacy of the peptidergic nootropic drug cerebrolysin in patients with senile dementia of the Alzheimer type (SDAT). Pharmacopsychiatry 27(1):32–40
Allegri RF, Guekht A (2012) Cerebrolysin improves symptoms and delays progression in patients with Alzheimer’s disease and vascular dementia. Drugs Today (Barc) 48(Suppl A):25–41
Gershman RN, Vasilenko MA (1975) Use of cerebrolysin and ATP in treating infantile cerebral paralysis. Pediatr Akus Ginekol (1):22–23 (Article in Ukrainian)
Petrukhin AS, Pylaeva OA (2014) Cerebrolysin in pediatric neurology practice. Zh Nevrol Psikhiatr Im S S Korsakova 114(1 Pt 2):75–80 (Article in Russian)
Hong Z, Moessler H, Bornstein N, Brainin M, Heiss WD, Investigators C (2009) A double-blind, placebo-controlled, randomized trial to evaluate the safety and efficacy of cerebrolysin in patients with acute ischaemic stroke in Asia–CASTA. Int J Stroke Off J Int Stroke Soc 4(5):406–412
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The protocol of the study was approved by the Pediatrics Review Board and the Regional Ethics Committee of Isfahan University of Medical Sciences (Research Project Number: 393470).
Informed consent
Written informed consent was obtained from the parents of selected patients after describing the goal of and treatments in the study.
Rights and permissions
About this article
Cite this article
Nasiri, J., Safavifar, F. Effect of cerebrolysin on gross motor function of children with cerebral palsy: a clinical trial. Acta Neurol Belg 117, 501–505 (2017). https://doi.org/10.1007/s13760-016-0743-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-016-0743-x