Abstract
Purpose
To explore the biliary and duodenal microbiota features associated with the formation and recurrence of choledocholithiasis (CDL).
Methods
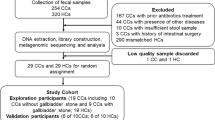
We prospectively recruited patients with primary (P-CDL, n = 29) and recurrent CDL (R-CDL, n = 27) for endoscopic retrograde cholangiopancreatography (ERCP). Duodenal mucosa (DM), bile and bile duct stones (BDS) samples were collected in P- and R-CDL patients. DM samples were also collected in 8 healthy controls (HC). The microbiota profile analysis was performed with 16S rRNA gene sequencing.
Results
Short-course antibiotic application before ERCP showed no significant effects in alpha and beta diversities of the biliary and duodenal microbiota in CDL. Alpha diversity showed no difference between DM and bile samples in CDL. The duodenal microbial richness and diversity was lower in both P- and R-CDL than HC. The biliary microbiota composition showed a high similarity between P- and R-CDL. Fusobacterium and Enterococcus were higher abundant in DM, bile, and BDS samples of R-CDL than P-CDL, as well as Escherichia and Klebsiella in bile samples of R-CDL. The enriched duodenal and biliary bacteria in CDL were closely associated with cholecystectomy, inflammation and liver dysfunction. The bile-associated microbiota of R-CDL expressed enhanced capacity of D-glucuronide and D-glucuronate degradation, implicating an elevated level of β-glucuronidase probably produced by enriched Escherichia and Klebsiella in bile.
Conclusions
The duodenal microbiota was in an imbalance in CDL. The duodenal microbiota was probably the main source of the biliary microbiota and was closely related to CDL formation and recurrence. Enterococcus, Fusobacterium, Escherichia and Klebsiella might contribute to CDL recurrence.
Clinical trials
The study was registered at the Chinese Clinical Trial Registry (https://www.chictr.org.cn/index.html, ChiCTR2000033940).






Similar content being viewed by others
References
Tazuma S. Gallstone disease: Epidemiology, pathogenesis, and classification of biliary stones (common bile duct and intrahepatic). Best Pract Res Clin Gastroenterol. 2006;20(6):1075–83.
Verdier J, Luedde T, Sellge G. Biliary mucosal barrier and microbiome. Viszeralmedizin. 2015;31(3):156–61.
Keren N, Konikoff FM, Paitan Y, et al. Interactions between the intestinal microbiota and bile acids in gallstones patients. Environ Microbiol Rep. 2015;7(6):874–80.
Cai JS, Qiang S, Bao-Bing Y. Advances of recurrent risk factors and management of choledocholithiasis. Scand J Gastroenterol. 2017;52(1):34–43.
Maki T. Pathogenesis of calcium bilirubinate gallstone: role of E. coli, beta-glucuronidase and coagulation by inorganic ions, polyelectrolytes and agitation. Ann Surg. 1966;164(1):90–100.
Leung JW, Liu YL, Leung PS, et al. Expression of bacterial beta-glucuronidase in human bile: an in vitro study. Gastrointest Endosc. 2001;54(3):346–50.
Stewart L, Grifiss JM, Jarvis GA, et al. Biliary bacterial factors determine the path of gallstone formation. Am J Surg. 2006;192(5):598–603.
dos Santos JS, Júnior WS, Módena JL, et al. Effect of preoperative endoscopic decompression on malignant biliary obstruction and postoperative infection. Hepatogastroenterology. 2005;52(61):45–7.
Ipek S, Alper E, Cekic C, et al. Evaluation of the effectiveness of endoscopic retrograde cholangiopancreatography in patients with perihilar cholangiocarcinoma and its effect on development of cholangitis. Gastroenterol Res Pract. 2014;20:508286.
Ye F, Shen H, Li Z, et al. Influence of the biliary system on biliary bacteria revealed by bacterial communities of the human biliary and upper digestive tracts. PLoS ONE. 2016;11(3): e0150519.
Zhang ZH, Wu SD, Wang B, et al. Sphincter of Oddi hypomotility and its relationship with duodenal-biliary reflux, plasma motilin and serum gastrin. World J Gastroenterol. 2008;14(25):4077–81.
Konstantakis C, Triantos C, Theopistos V, et al. Recurrence of choledocholithiasis following endoscopic bile duct clearance: long term results and factors associated with recurrent bile duct stones. World J Gastrointest Endosc. 2017;9(1):26–33.
Zhang Q, Ye M, Su W, et al. Sphincter of Oddi laxity alters bile duct microbiota and contributes to the recurrence of choledocholithiasis. Ann Transl Med. 2020;8(21):1383.
Dumonceau JM, Kapral C, Aabakken L, et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2020;52(2):127–49.
Cheon YK, Lehman GA. Identification of risk factors for stone recurrence after endoscopic treatment of bile duct stones. Eur J Gastroenterol Hepatol. 2006;18(5):461–4.
Liang T, Su W, Zhang Q, et al. Roles of sphincter of Oddi laxity in bile duct microenvironment in patients with Cholangiolithiasis: from the perspective of the Microbiome and Metabolome. J Am Coll Surg. 2016;222(3):269-280.e210.
Liang TB, Liu Y, Bai XL, et al. Sphincter of Oddi laxity: an important factor in hepatolithiasis. World J Gastroenterol. 2010;16(8):1014–8.
Mu H, Gao J, Kong Q, et al. Prognostic factors and postoperative recurrence of calculus following small-incision sphincterotomy with papillary balloon dilation for the treatment of intractable choledocholithiasis: a 72-month follow-up study. Dig Dis Sci. 2015;60(7):2144–9.
Kawaji Y, Isayama H, Nakai Y, et al. Multiple recurrences after endoscopic removal of common bile duct stones: a retrospective analysis of 976 cases. J Gastroenterol Hepatol. 2019;34(8):1460–6.
Choi JH, Seo DW. Reappraisal of duodenobiliary reflux in bile duct stone recurrence: more than just reflux. Gastrointest Endosc. 2015;82(4):666–7.
Leung JW, Sung JY, Costerton JW. Bacteriological and electron microscopy examination of brown pigment stones. J Clin Microbiol. 1989;27(5):915–21.
Ye C, Zhou W, Zhang H, et al. Alterations of the bile microbiome in recurrent common bile duct stone. Biomed Res Int. 2020;20:4637560.
Chen B, Fu SW, Lu L, et al. A Preliminary study of biliary microbiota in patients with bile duct stones or distal cholangiocarcinoma. Biomed Res Int. 2019;20:1092563.
Lee J, Park JS, Bae J, et al. Bile microbiome in patients with recurrent common bile duct stones and correlation with the duodenal microbiome. Life (Basel). 2022;12:10.
Han J, Wu S, Fan Y, et al. Biliary microbiota in choledocholithiasis and correlation with duodenal microbiota. Front Cell Infect Microbiol. 2021;11:625589.
Nzenza TC, Al-Habbal Y, Guerra GR, et al. Recurrent common bile duct stones as a late complication of endoscopic sphincterotomy. BMC Gastroenterol. 2018;18(1):39.
Kim KY, Han J, Kim HG, et al. Late complications and stone recurrence rates after bile duct stone removal by endoscopic sphincterotomy and large balloon dilation are similar to those after endoscopic sphincterotomy alone. Clin Endosc. 2013;46(6):637–42.
Choe JW, Lee JM, Hyun JJ, et al. Analysis on microbial profiles & components of bile in patients with recurrent cbd stones after endoscopic cbd stone removal: a preliminary study. J Clin Med. 2021;10:15.
Saharia PC, Zuidema GD, Cameron JL. Primary common duct stones. Ann Surg. 1977;185(5):598–604.
Hall M, Beiko RG. 16S rRNA Gene Analysis with QIIME2. Methods Mol Biol. 2018;1849:113–29.
Chong J, Liu P, Zhou G, et al. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat Protoc. 2020;15(3):799–821.
Douglas GM, Maffei VJ, Zaneveld JR, et al. PICRUSt2 for prediction of metagenome functions. Nat Biotechnol. 2020;38(6):685–8.
Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60.
Mori AS, Furukawa T, Sasaki T. Response diversity determines the resilience of ecosystems to environmental change. Biol Rev Camb Philos Soc. 2013;88(2):349–64.
Molinero N, Ruiz L, Milani C, et al. The human gallbladder microbiome is related to the physiological state and the biliary metabolic profile. Microbiome. 2019;7(1):100.
Yasuda I, Fujita N, Maguchi H, et al. Long-term outcomes after endoscopic sphincterotomy versus endoscopic papillary balloon dilation for bile duct stones. Gastrointest Endosc. 2010;72(6):1185–91.
Shen H, Ye F, Xie L, et al. Metagenomic sequencing of bile from gallstone patients to identify different microbial community patterns and novel biliary bacteria. Sci Rep. 2015;5:17450.
Nomura T, Shirai Y, Hatakeyama K. Enterococcal bactibilia in patients with malignant biliary obstruction. Dig Dis Sci. 2000;45(11):2183–6.
Lipsett PA, Pitt HA. Acute cholangitis. Surg Clin North Am. 1990;70(6):1297–312.
Kim B, Park JS, Bae J, et al. Bile microbiota in patients with pigment common bile duct stones. J Korean Med Sci. 2021;36(15): e94.
Kose SH, Grice K, Orsi WD, et al. Metagenomics of pigmented and cholesterol gallstones: the putative role of bacteria. Sci Rep. 2018;8(1):11218.
Brennan CA, Garrett WS. Fusobacterium nucleatum - symbiont, opportunist and oncobacterium. Nat Rev Microbiol. 2019;17(3):156–66.
Li Y, Tan WH, Wu JC, et al. Microbiologic risk factors of recurrent choledocholithiasis post-endoscopic sphincterotomy. World J Gastroenterol. 2022;28(12):1257–71.
Gomi H, Solomkin JS, Schlossberg D, et al. Tokyo Guidelines 2018: antimicrobial therapy for acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2018;25(1):3–16.
Grigoreva IN, Romanova TI. Gallstone disease and microbiome. Microorganisms. 2020;8:6.
Awolade P, Cele N, Kerru N, et al. Therapeutic significance of β-glucuronidase activity and its inhibitors: a review. Eur J Med Chem. 2020;187:111921.
Chen X, Yan XR, Zhang LP. Ursodeoxycholic acid after common bile duct stones removal for prevention of recurrence: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2018;97(45): e13086.
Yoon WJ, Kim HN, Park E, et al. The impact of cholecystectomy on the gut microbiota: a case-control study. J Clin Med. 2019;8(1):58.
Serra N, Di Carlo P, D’Arpa F, et al. Human bile microbiota: a retrospective study focusing on age and gender. J Infect Public Health. 2021;14(2):206–13.
Acknowledgements
Thanks to Chenhuan Wang, Lijuan Xu, Hong Du, and Ting Yang for helping with sample collection.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
LF, WZ, XG, and LW designed the study; XG and LF analyzed the omics data and created figures; LF, LM, ZX, CF, and WZ collected and interpreted the clinical data; LF and WZ drafted the manuscript; GX and LW revised the manuscript; all authors read and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
The study was approved by the Ethics Committee of the PLA General Hospital (S-2019-274-01).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13755_2023_267_MOESM1_ESM.tiff
Assessment of the effect of antibiotic prophylaxis on the duodenal and biliary microbiota diversity and composition in patients with CDL. Comparisons of the (A-C) alpha diversity (Choa1 index and Shannon index) and (D-F) beta diversity between patients with (ABX) and without (None) antibiotic prophylaxis treatment. Significance of comparing the alpha diversity was determined by the Wilcoxon rank sum test. Significance of Bray–Curtis dissimilarity was tested by PERMANOVA. Supplementary file1 (TIFF 24519 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, F., Wang, ZK., Li, MY. et al. Characterization of biliary and duodenal microbiota in patients with primary and recurrent choledocholithiasis. Health Inf Sci Syst 12, 29 (2024). https://doi.org/10.1007/s13755-023-00267-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13755-023-00267-2