Abstract
Constipation is the most common digestive complaint in the general population. Anthranoid laxatives are easily available, not expensive and are among the most sold drugs, but no randomized placebo-controlled studies have been performed to assess their effectiveness and safety in patients with chronic constipation.
Methods/Aims
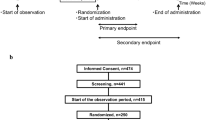
Aim of this study was to evaluate the effect of once-daily administration of an infusion prepared using a commercially available mixture of herbs containing anthranoid laxatives (HAM: herbal anthranoid mixture) taken in the evening before bedtime for 10 days in patients with chronic constipation. We conducted a double-blind randomized placebo-controlled cross-over study, on patients with chronic constipation according with the Roma III criteria. The patients were randomly divided (1:1) into two arms, according to a cross-over protocol. Arm A received a 10 days treatment (HAM), no therapy for 10 days and placebo for other 10 days. Arm B received placebo for 10 days, no therapy for 10 days and HAM for the other 10 days.
Results
A significant difference (p<0.001) was found between HAM and placebo periods regarding stool frequency after ten days of treatment [mean difference of 5.05 (95% CI: 3.5–6.49)]. Only one patient in arm A discontinued the treatment after three days for the appearance of watery diarrhea that disappeared in 24 hours.
Conclusion
In this double-blind randomized placebo-controlled cross-over pilot study, HAM seems to offer an advantage in chronic constipation improving stool frequency without important side effects, showing that anthranoid laxatives are effective and safe.
Similar content being viewed by others
References
Higgins PD, Johanson JF (2004) Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol 99:750–759
Lembo A, Camilleri M (2009) Chronic constipation. N Engl J Med 349:1360–1368
Pare P, Ferrazzi S, Thompson WG et al (2001) An epidemiological survey of constipation in Canada: definitions, rates, demographics and predictors of health care seeking. Am J Gastroenterol 96:3130–3137
Johanson JF, Kralstein J (2007) Chronic constipation: a survey of the patients perspective. Aliment Pharmacol Ther 25:599–608
Wald A, Scarpignato C, Kamm MA et al (2007) The burden of constipation on quality of life: result of a multinational survey. Aliment Pharmacol Ther 26:227–236
Martin BC, Barghout V, Cerulli A (2006) Direct medical cost of constipation in the United States. Manag Care Interface 19:43–49
Talley NJ (2008) Functional gastrointestinal disorders as a public health problem. Neurogastroenterol Motil 20[Suppl 1]:121–129
Brandt LJ, Prather CM, Quigley EM et al (2005) Systematic review of chronic constipation in North America. Am J Gastroenterol 100[Suppl 1]:S5–S21
Sweeney M (1997) Constipation diagnosis and treatment. Home Care Provider 2:250–255
Tramonte SM, Brand MB, Mulrow CD et al (1997) The treatment of chronic constipation in adults: a systematic review. J Gen Intern Med 12:15–24
Franz G (1993) The senna drug and its chemistry. Pharmacology 47[Suppl 1]:2–6
Brown JP (1980) A review of the genetics effects of naturally occurring flavonoids, anthraquinones and related compounds. Mutat Res 75:243–277
Van Os FHL (1976) Anthraquinone derivates in vegetable laxatives. Pharmacology 14[Suppl 1]:7–17
Leng Peschlow E (1986) Dual effect of orally administrated sennosides on large intestine transit and fluids absorption in the rat. J Pharm Pharmacol 38:606–610
Ewe K (1980) Effect of rhein on the transport of electrolytes, water and carbohydrates in the human jejunum and colon. Pharmacology 20:27–35
Marlett JA, Li BU, Patrow CJ, Bass P (1987) Comparative laxation of psyllium with and without senna in an ambulatory constipated population. Am J Gastroenterol 82:333–337
MacLennan WJ, Pode AFWM (1975) A comparison of sodium picosulphatec (Laxoberal) with standardised senna (Senokot) in geriatric patients. Curr Med Res Opin 2:641–647
Cummings JH (1974) Laxative abuse. Gut 15:758–766
Rawson MD (1966) Cathartic colon. Lancet i:1121–1124
Marshak RH, Gerson A (1960) Cathartic colon. Am J Dig Dis 5:724–727
Avery-Jones F (1967) Cathartic colon. Proc R Soc Med 60:503–504
Smith B (1968) Effect of irritant purgatives on the myenteric plexus in man and the mouse. Gut 9:139–143
Smith B (1973) Pathologic changes in the colon produced by anthraquinone purgatives. Dis Colon Rectum 16:455–458
Kiernan JA, Heinicke EA (1989) Sennosides do not kill myenteric neurons in the colon of the rat or mouse. Neuroscience 30:837–842
Dufour P, Gendre P (1984) Ultrastructure of mouse intestinal mucosa and changes observed after long term anthraquinone administration. Gut 25:1358–1363
Speare GS (1951) Melanosis coli: experimental observations on its production and elimination in 23 cases. Am J Surg 82:631–637
Bockus HI, Willard JH, Banks J (1933) Melanosis coli. The etiologic significance of the anthracene laxatives: a report of forty-one cases. J Am Med Assoc 101:1–6
Steer HW, Colin-Jones DG (1975) Melanosis coli: studies of the toxic effect of irritant purgatives. J Pathol 115:199–205
Zobel AJ, Susnow DA (1935) Melanosis coli: its clinical significance. Arch Surg 30:974–979
Wittoesch JH, Jackman RJ, McDonald JR (1958) Melanosis coli: general review and a study of 887 cases. Dis Colon Rectum 1:172–180
Badiali D, Marcheggiano A, Pallone F et al (1985) Melanosis of the rectum in patients with chronic constipation. Dis Colon Rectum 28:241–245
Leng-Peschlow E (1992) Senna and pseudomelanosis coli. Pharmacology 44[Suppl 1]:33–35
Boyd JT, Doll R (1954) Gastro-intestinal cancer and use of liquid paraffin. Br J Cancer 8:231–237
Nakamura GJ, Schneiderman LJ, Klauber MR (1984) Colorectal cancer and bowel habits. Cancer 54:1475–1477
Johnson JF, Karlstein J (2007) Chronic constipation: a survey of patients perspective. Aliment Pharmacol Ther 25:599–608
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tari, R., Carmagnola, S., Pagliarulo, M. et al. Are anthranoid laxatives effective in chronic constipation?. Nutrafoods 11, 131–136 (2012). https://doi.org/10.1007/s13749-012-0052-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13749-012-0052-9