Abstract
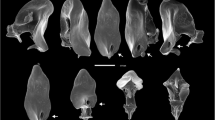
In this work, we investigated the morphological variation of the intromittent male copulatory organ (aedeagus) of specimens from natural populations of two cactophilic Drosophila species distributed in the southeast region of Brazil, Drosophila gouveai Tidon-Sklorz & Sene and Drosophila antonietae Tidon-Sklorz & Sene. It was explored how the within-species variability is arranged for both species, considering their historical and ecological features. Our results showed two distinct aedeagal morphologies for these species, and differences within species were observed only in D. gouveai as specimens could be distinguished by their population origin. In contrast, after size discrepancies correction, this feature was not detected in D. antonietae. The contrasting patterns of intraspecific variation, together with the other features exhibited by these two species, are most likely to be explained by differences in the historical host plant association and distribution and in demographic events, which determined the evolutionary history of these two South American cactophilic Drosophila species.



Similar content being viewed by others
References
Ab’Saber AN (1977) Os domínios morfoclimáticos da América do Sul. Geomorfologia 52:1–21
Ab’Saber AN (2000) Spaces occupied by the expansion of dry climates in South America during the Quaternary ice ages. Rev I Geol 21:71–78
Andrade CAC, Vieira RD, Ananina G, Klaczko LB (2009) Evolution of the male genitalia: morphological variation of the aedeagi in a natural population of Drosophila mediopunctata. Genetica 135:13–23
Barrowclough GF, Flesness NR (1996) Species, subspecies, and races: the problem of units of management in conservation. In: Kleiman DG, Allen ME, Thompson KV, Lumpkin S, Harris H (eds) Wild mammal in captivity: principles and techniques. University of Chicago Press, Chicago, pp 247–254
Bigarella JJ, Andrade-Lima D, Riehs PJ (1975) Considerações a respeito das mudanças paleoclimáticas na distribuição de algumas espécies vegetais e animais no Brasil. An Acad Bras Cienc 41:411–464
Brown JH (1984) On the relationship between abundance and distribution of species. Am Nat 124:255–279
Carreira VP, Soto IM, Hasson E, Fanara JJ (2006) Patterns of variation in wing morphology in the cactophilic Drosophila buzzatii and its sibling D. koepferae. J Evol Biol 9:1275–1282
Carreira VP, Soto IM, Fanara JJ, Hasson E (2008) A study of wing morphology and fluctuating asymmetry in interspecific hybrids between Drosophila buzzatii and D. koepferae. Genetica 133:1–11
De Brito RA, Manfrin MH, Sene FM (2002) Nested cladistic analysis of Brazilian populations of Drosophila serido. Mol Phylogenet Evol 22:131–143
DeSalle R, Grimaldi DA (1991) Morphological and molecular systematics of the Drosophilidae. Annu Rev Ecol Syst 22:447–475
Eberhard WG (2010) Rapid divergent evolution of genitalia: theory and data updated. In: Leonard JL, Córdoba-Aguilar A (eds) The Evolution of Primary Sexual Characters in Animals. Oxford University Press, New York, pp 40–78
Falconer DS (1989) Introduction to quantitative genetics, 2nd edn. Longman, London, 438 p
Fanara JJ, Fontdevila A, Hasson E (1999) Oviposition preference, viability, developmental time and body size in the cactophilic sibling species Drosophila koepferae and D. buzzatii in association to their natural hosts. Evol Ecol 13:173–190
Fanara JJ, Mensch J, Folguera G, Hasson E (2004) Developmental time and thorax length differences between the cactophilic species Drosophila buzzatii and D. koepferae reared in different natural hosts. Evol Ecol 18:203–214
Fanara JJ, Folguera G, Fernández-Iriarte P, Mensch J, Hasson E (2006) Genotype by environment interactions in viability and developmental time in populations of cactophilic Drosophila. J Evolution Biol 9:900–908
Franco FF, Prado PRR, Sene FM, Costa LF, Manfrin MH (2006) Aedeagus morphology as a discriminant marker in two closely related cactophilic species of Drosophila (Diptera; Drosophilidae) in South America. An Acad Bras Cienc 78:203–212
Franco FF, Soto IM, Sene FM, Manfrin MH (2008) Phenotypic variation of the aedeagus of Drosophila serido Vilela & Sene (Diptera: Drosophilidae). Neotrop Entomol 37:558–563
Garnier S, Magniez-Jannin F, Rasplus JY, Alibert P (2005) When morphometry meets genetics: inferring the phylogeography of Carabus solieri using Fourier analyses of pronotum and male genitalia. J Evolution Biol 18:269–280
Gilchrist AS, Partridge L (1999) A comparison of the genetic basis of wing size divergence in three parallel body size clines of Drosophila melanogaster. Genetics 153:1775–1787
Gilchrist GW, Huey RB, Serra L (2002) Rapid evolution of wing size clines in Drosophila subobscura. In: Hendry AP, Kinnison MT (eds) Microevolution rate, pattern, process: contemporary issues in genetics and evolution. Springer, Netherlands, pp 273–286
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:9p. http://palaeo-electronica.org/2001_1/past/issue1_01.htm
Holbrook FR, Tabachnick WJ, Schmidtmann ET, Mckinnon CN, Bobian RJ, Grogan WL (2000) Sympatry in the Culicoides variipennis Complex (Diptera: Ceratopogonidae): a taxonomic reassessment. J Med Entomol 37:65–76
Holzschu PL, Phaff HF (1982) Taxonomy and evolution of some ascomycetous cactophilic yeast. In: Barker JSF, Starmer WT (eds) Ecological genetics and evolution: the cactus–yeast–Drosophila model system. Academic Press, New York, pp 127–141
James FC, McCulloch CE (1990) Multivariate analysis in ecology and systematics: panacea or pandora's box? Annu Rev Ecol Syst 21:129–166
Johnston JS, Heed WB (1976) Dispersal of desert-adapted Drosophila: the saguaro-breeding D. nigrospiracula. Am Nat 110:629–651
Johnston JS, Templeton AR (1982) Dispersal and clines in Opuntia breeding Drosophila mercatorum and D. hydei at Kamuela, Hawaii. In: Barker JSF, Starmer WT (eds) Ecological genetics and evolution: the cactus–yeast–Drosophila model system. Academic Press, Sydney, pp 241–256
Kaneshiro KY (1969) A study of the relationship of Hawaiian Drosophila species based on external male genitalia. U Texas Publ 6918:55–70
Killick-Kendrick R, Tang Y, Killick-Kendrick M, Johnson RN, Ngumbi PM, Sang DK, Lawyer PG (1994) Phlebotomine sandflies of Kenya (Diptera: Psychodidae). III: the identification and distribution of species of the subgenus Larroussius. Ann Trop Med Parasit 88:183–196
Kullikov AM, Melnikov AI, Gornostaev NG, Lazebny OE, Mitrofanov VG (2004) Morphological analysis of male mating organ in the Drosophila virilis species group: a multivariate approach. J Zool Syst Evol Res 42:135–144
Liu J, Merger JM, Stam LF, Gibson GC, Zeng ZB, Laurie CC (1996) Genetic analysis of a morphological shape difference in the male genitalia of Drosophila simulans and D. mauritiana. Genetics 142:1129–1145
Machado LPB, Mateus RP, Sene FM, Manfrin MH (2010) Microsatellite allele sequencing in population analyses of the South American cactophilic species Drosophila antonietae (Diptera: Drosophilidae). Biol J Linn Soc 100:573–584
Manfrin MH, Sene FM (2006) Cactophilic Drosophila in South America: a model for evolutionary studies. Genetica 126:57–75
Manfrin MH, De Brito ROA, Sene FM (2001) Systematics and evolution of Drosophila buzzatii cluster using mtDNA. Ann Entomol Soc Am 94:333–346
Manly BFJ (1994) Multivariate statistical methods: a primer, 2nd edn. Chapman & Hall, London, 215 p
Markow TA, Castrezana S (2000) Dispersal in cactophilic Drosophila. Oikos 89:378–386
Mardia KV, Kent JT, Bibby JM (1979) Multivariate analysis. Academic Press, New York, 521 p
Mateus RP, Sene FM (2003) Temporal and spatial allozyme variation in the South American cactophilic Drosophila antonietae (Diptera, Drosophilidae). Biochem Genet 41:219–233
Mateus RP, Sene FM (2007) Population genetic study of allozyme variation in natural populations of Drosophila antonietae (Insecta, Diptera). J Zool Syst Evol Res 45:136–143
Mateus RP, Buschini MLT, Sene FM (2006) The Drosophila community in xerophytic vegetations of the upper Parana–Paraguay River Basin. Braz J Biol 66:719–729
Mateus RP, Machado LPB, Moraes EM, Sene FM (2010) Allozymatic divergence between border populations of two cryptic species of the Drosophila buzzatii cluster species (Diptera: Drosophilidae). Biochem Syst Ecol 38:410–415
Matzkin LM, Watts TD, Bitler BG, Machado CA, Markow TA (2006) Functional genomics of cactus host shifts in Drosophila mojavensis. Mol Ecol 15:4635–4643
Monteiro LR, Diniz-Filho JAF, dos Reis SF, Araújo ED (2002) Geometric estimates of heritability in biological shape. Evolution 56:563–572
Monteiro SG, Sene FM (1995) Estudo morfométrico de populações de Drosophila serido das regiões Central e Sul do Brasil. Braz J Genet 18:283–283
Moraes EM, Sene FM (2002) Breeding structure of an isolated cactophilic Drosophila population on a sandstone table hill. J Zool Syst Evol Res 40:123–128
Moraes EM, Sene FM (2007) Microsatellite and morphometric variation in Drosophila gouveai: the relative importance of historical and current factors in shaping the genetic population structure. J Zool Syst Evol Res 45:336–344
Morais PB, Rosa CA, Hagler NA, Mendonça-Hagler LC (1994) Yeast communities of the cactus Pilosocereus arrabidae as resources for larval and adult stages of Drosophila serido. Anton Van Leeuw 66:313–317
Moreteau B, Gibert P, Pétavy G, Moereteau JC, Huey RB, David JR (2003) Morphometrical evolution in a Drosophila clade: the Drosophila obscure group. J Zool Syst Evol Res 41:64–71
Morrone JJ (2006) Biogeographic areas and transition zones of Latin America and the Caribbean islands based on panbiogeographic and cladistic analyses of the entomofauna. Annu Rev Entomol 51:467–494
Morrone JJ, Mazzucconi SA, Bachmann AO (2004) Distributional patterns of Chacoan water bugs (Heteroptera: Belostomatidae, Corixidae, Micronectidae and Gerridae). Hydrobiologia 523:159–173
Neff WA, Marcus LF (1980) A survey of multivariate methods for systematics. American Museum of Natural History, New York, p 221
Prado DE, Gibbs PE (1993) Patterns of species distributions in the dry seasonal forests of South America. Ann MO Bot Gard 80:902–927
Prado PRP, Franco FF, Manfrin MH, Costa LF, Sene FM (2004) An easy and fast way to analyze morphometric characters. Proc Third Braz Symp Mathem Comp Biol 1:329–340
Rice WR (1985) Disruptive selection on habitat preference and the evolution of reproductive isolation: an exploratory experiment. Evolution 39:645–656
Rice WR, Hostert EE (1993) Laboratory experiments on speciation: what have we learned in 40 Years? Evolution 47:1637–1653
Rohlf FJ (2000) NTSYS 2.1: numerical taxonomic and multivariate analysis system. Exeter Software, New York
Rohlf FJ, Bookstein FL (1987) A comment on shearing as a method for “size correction”. Syst Zool 36:356–367
Ruiz A, Heed WB (1988) Host-plant specify in the cactophilic Drosophila mulleri species complex. J Anim Ecol 57:237–249
Sene FM, Val FC, Vilela CR, Pereira MAQR (1980) Preliminary data on the geographical distribution of Drosophila species within morphoclimatic domains of Brazil. Pap Av Dep Zool Sec Agric S Paulo 33:315–326
Sene FM, Pereira MAQR, Vilela CR, Bizzo NMV (1981) Influence of different ways to set baits for collection of Drosophila flies in three natural environments. Dros Inf Serv 56:118–121
Silva AFG, Sene FM (1991) Morphological geographic variability in Drosophila serido (Diptera, Drosophilidae). Braz J Entomol 35:455–468
Soto IM, Carreira VP, Fanara JJ, Hasson ER (2007a) Evolution of male genitalia: environmental and genetic factors affect genital morphology in two Drosophila sibling species and their hybrids. BMC Evol Biol 7:77–87
Soto IM, Manfrin MH, Sene FM, Hasson E (2007b) Viability and developmental time in cactophilic Drosophila gouveai and Drosophila antonietae (Diptera: Drosophilidae) are dependent on the cactus host. Ann Entomol Soc Am 100:490–496
Soto IM, Manfrin MH, Hasson E (2008a) Host-dependent phenotypic plasticity of aedeagus morphology in a pair of cactophilic sibling Drosophila species of the repleta group (Diptera, Drosophilidae). J Zool Syst Evol Res 46:368–373
Soto EM, Soto IM, Carreira VP, Fanara JJ, Hasson E (2008b) Host-related life history traits in interspecific hybrids of cactophilic Drosophila. Entomol Exp Appl 126:18–27
Soto IM, Carreira VP, Soto EM, Hasson E (2008c) Wing morphology and fluctuating asymmetry depend on the host plant in cactophilic Drosophila. J Evol Biol 21:598–609
Spichiger R, Calenge C, Bise B (2004) Geographical zonation in the Neotropics of tree species characteristic of the Paraguay-Parana Basin. J Biogeogr 31:1489–1501
Starmer WT (1982) Associations and interactions among yeasts, Drosophila and their habitats. In: Barker JSF, Starmer WT (eds) Ecological genetics and evolution: the cactus–yeast–Drosophila model system. Academic Press, Sydney, pp 159–174
Taylor N, Zappi D (2004) Phytogeography. In: Taylor N, Zappi D (eds) Cacti of Eastern Brazil. Kew, Royal Botanic Gardens, pp 35–138
Templeton AR, Johnston JS (1982) Life history evolution under pleiotropy and K-selection in a natural population of Drosophila mercatorum. In: Barker JSF, Starmer WT (eds) Ecological genetics and evolution: the cactus–yeast–Drosophila model system. Academic Press, Sydney, pp 225–239
Tidon-Sklorz R, Sene FM (1995a) Drosophila seriema: a new member of the Drosophila serido (Diptera, Drosophilidae) superspecies taxon. Ann Entomol Soc Am 88:139–142
Tidon-Sklorz R, Sene FM (1995b) Evolution of the buzzatii cluster (Drosophila repleta species group) in middle South America. Evol Biol 8(9):71–85
Tidon-Sklorz R, Sene FM (2001) Two new species of the Drosophila serido sibling set (Diptera, Drosophilidae). Iheringia, Sér Zool 90:141–146
Vilela CR (1983) A revision of the Drosophila repleta species group (Diptera, Drosophilidae). Braz J Entomol 27:1–114
Vujic’ A, Stahl G, Rojo S, Radenkovic’ S, Šimic’ S (2008) Systematics and phylogeny of the tribe Paragini (Diptera: Syrphidae) based on molecular and morphological characters. Zool J Linn Soc 152:507–536
Wheeler MR, Kambysellis MP (1966) Notes on the Drosophilidae (Diptera) of Samoa. Univ Texas Publ 6615:533–565
Acknowledgements
We would like to thank the three anonymous referees for the valid and useful suggestions. This research was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Universidade de São Paulo (USP).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Roberto A Zucchi – ESALQ/USP
Rights and permissions
About this article
Cite this article
Mateus, R.P., Moura, M.O., Manfrin, M.H. et al. Contrasting Patterns of Within-Species Morphological Variation in Two Cactophilic Drosophila Species (Diptera: Drosophilidae). Neotrop Entomol 42, 384–392 (2013). https://doi.org/10.1007/s13744-013-0128-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-013-0128-2