Abstract
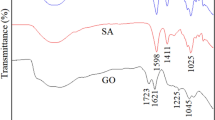
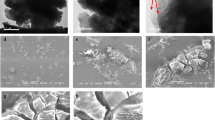
This study involves the preparation of sodium alginate poly grafted (fumaric acid-polyacrylic acid)/graphene oxide, SA-g-p(FA-AA)/GO hydrogel to explore its potential as a promising adsorbent for water treatment mainly chromium (VI) and lead (II) removal. Prepared adsorbent was characterized by FTIR, TGA, XRD, FESEM, and TEM techniques for exploring the chemical structure, thermal stability, crystallography, surface area and morphology, as well as pore size and distribution of SA-g-p(FA-AA)/GO, respectively. The average size of the prepared nanoparticles was observed to be 78.48 nm. The TEM images exhibit a predominantly spherical shape and heterogeneous. Effect of different physiochemical parameters such as pH, temperature, adsorbent dosage, and contact time was explored for maximum metal adsorption. The results of the study revealed that the maximum adsorption capacity of SA-g-p(FA-AA)/GO (0.045 mg g−1 for Cr (VI) and 22.371 mg g−1 for Pb (II)) was achieved under optimized conditions, i.e., adsorbent dose of 0.05 g at 25 °C for pH of 2, 4.5 when contact time of 5 and 100 min was used for Cr(VI) and Pb(II), respectively. Data fits best to the pseudo-second-order kinetic equation revealing the multilayer adsorption of Cr (VI) and Pb (II) ions on the heterogeneous adsorbent surface. Thermodynamically, the process of Cr (VI) and Pb (II) adsorption was non-spontaneous, exothermic and feasible revealing the potential of the prepared adsorbent to be used as an efficient adsorbent for metal removal.










Similar content being viewed by others
References
A. Azimi, A. Azari, M. Rezakazemi, M. Ansarpour, Removal of heavy metals from industrial wastewaters: a review. ChemBioEng Rev. 4, 37–59 (2017)
G. Palani, A. Arputhalatha, K. Kannan et al., Current trends in the application of nanomaterials for the removal of pollutants from industrial wastewater treatment—a review. Molecules 26, 2799 (2021)
H. Souhassou, Y. Fahoul, I. El Mrabet, et al., Optimization of basic red 29 dye removal onto a natural red clay using response surface methodology. J. Iranian Chem. Soc., 1–17 (2023)
M.B. Poudel, M. Shin, H.J. Kim, Interface engineering of MIL-88 derived MnFe-LDH and MnFe2O3 on three-dimensional carbon nanofibers for the efficient adsorption of Cr (VI), Pb (II), and As (III) ions. Sep. Purif. Technol. 287, 120463 (2022)
S. Water and W. H. Organization, Guidelines for drinking-water quality [electronic resource]: incorporating first addendum. Vol. 1, Recommendations. (2006)
F. Edition, Guidelines for drinking-water quality. WHO Chron. 38, 104–108 (2011)
S. Rajendran, T. Priya, K.S. Khoo et al., A critical review on various remediation approaches for heavy metal contaminants removal from contaminated soils. Chemosphere 287, 132369 (2022)
P. Punia, M.K. Bharti, R. Dhar et al., Recent advances in detection and removal of heavy metals from contaminated water. ChemBioEng Rev. 9, 351–369 (2022)
N.A. Qasem, R.H. Mohammed, D.U. Lawal, Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water 4, 36 (2021)
H.S. Ibrahim, N.S. Ammar, M. Soylak, M. Ibrahim, Removal of Cd (II) and Pb (II) from aqueous solution using dried water hyacinth as a biosorbent. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 96, 413–420 (2012)
S. Rajendran, A. Priya, P.S. Kumar et al., A critical and recent developments on adsorption technique for removal of heavy metals from wastewater-A review. Chemosphere 303, 135146 (2022)
A. Maftouh, O. El Fatni, S. El Hajjaji et al., Comparative review of different adsorption techniques used in heavy metals removal in water. Biointerface Res. Appl. Chem 13, 397 (2023)
H. Zhu, S. Chen, Y. Luo, Adsorption mechanisms of hydrogels for heavy metal and organic dyes removal: A short review. J. Agric. Food Res. 12, 100552 (2023)
Z. Darban, S. Shahabuddin, R. Gaur et al., Hydrogel-based adsorbent material for the effective removal of heavy metals from wastewater: a comprehensive review. Gels 8, 263 (2022)
M.A. Vafaei, A. Shakeri, H. Salehi et al., Cellulose nanofiber modified poly (Acrylic Acid-Co-N-Vinyl Pyrrolidone) hydrogel as forward osmosis draw agent. J. Polym. Environ. 31(10), 4369–4381 (2023)
X. Pei, L. Gan, Z. Tong et al., Robust cellulose-based composite adsorption membrane for heavy metal removal. J. Hazard. Mater. 406, 124746 (2021)
P.B. Vilela, A. Dalalibera, E.C. Duminelli et al., Adsorption and removal of chromium (VI) contained in aqueous solutions using a chitosan-based hydrogel. Environ. Sci. Pollut. Res. 26, 28481–28489 (2019)
P. Santander, B. Butter, E. Oyarce et al., Lignin-based adsorbent materials for metal ion removal from wastewater: A review. Ind. Crops Prod. 167, 113510 (2021)
X. Gao, C. Guo, J. Hao et al., Adsorption of heavy metal ions by sodium alginate based adsorbent-a review and new perspectives. Int. J. Biol. Macromol. 164, 4423–4434 (2020)
S. Thakur, B. Sharma, A. Verma et al., Recent progress in sodium alginate based sustainable hydrogels for environmental applications. J. Clean. Prod. 198, 143–159 (2018)
N. Mohanty, B.N. Patra, Polypyrrole-sodium alginate nanocomposites for enhanced removal of toxic organic and metal pollutants from wastewater. Mater. Today Commun. 34, 105325 (2023)
S. Velusamy, A. Roy, S. Sundaram, T. Kumar Mallick, A review on heavy metal ions and containing dyes removal through graphene oxide-based adsorption strategies for textile wastewater treatment. Chem. Record 21, 1570–1610 (2021)
K.Z. Elwakeel, M.M. Ahmed, A. Akhdhar et al., Recent advances in alginate-based adsorbents for heavy metal retention from water: a review. Desalin. Water Treat. 272, 50–74 (2022)
D. Thirumoolan, T. Siva, R. Ananthakumar, K.S.N. Nambi, Alginate-Based Superabsorbents, in Bio-based superabsorbents: recent trends, types, applications and recycling. ed. by S. Pradhan, S. Mohanty (Springer Nature Singapore, Singapore, 2023), pp.93–114
A. El Idrissi, A. El Gharrak, G. Achagri et al., Synthesis of urea-containing sodium alginate-g-poly (acrylic acid-co-acrylamide) superabsorbent-fertilizer hydrogel reinforced with carboxylated cellulose nanocrystals for efficient water and nitrogen utilization. J. Environ. Chem. Eng. 10, 108282 (2022)
K.A. Uyanga, Y. Iamphaojeen, W.A. Daoud, Effect of zinc ion concentration on crosslinking of carboxymethyl cellulose sodium-fumaric acid composite hydrogel. Polymer 225, 123788 (2021)
Z. Li, J. Shen, H. Ma et al., Preparation and characterization of pH-and temperature-responsive nanocomposite double network hydrogels. Mater. Sci. Eng. C 33, 1951–1957 (2013)
H. Jiang, Y. Yang, Z. Lin et al., Preparation of a novel bio-adsorbent of sodium alginate grafted polyacrylamide/graphene oxide hydrogel for the adsorption of heavy metal ion. Sci. Total Environ. 744, 140653 (2020)
J. Tang, J. Huang, T. Tun et al., Cu (II) and Cd (II) capture using novel thermosensitive hydrogel microspheres: adsorption behavior study and mechanism investigation. J. Chem. Technol. Biotechnol. 96, 2382–2389 (2021)
S. Tang, J. Yang, L. Lin et al., Construction of physically crosslinked chitosan/sodium alginate/calcium ion double-network hydrogel and its application to heavy metal ions removal. Chem. Eng. J. 393, 124728 (2020)
M. Tally, Y. Atassi, Synthesis and characterization of pH-sensitive superabsorbent hydrogels based on sodium alginate-g-poly (acrylic acid-co-acrylamide) obtained via an anionic surfactant micelle templating under microwave irradiation. Polym. Bull. 73, 3183–3208 (2016)
Acknowledgements
The authors would like to acknowledge the Research Council of University of Tabriz for their help and support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alnasery, H., Naseri, A., Jasim, L.S. et al. Synthesis, characterization, and adsorption capacity of sodium alginate poly grafted (fumaric acid-co-polyacrylic acid)/graphene oxide hydrogel as adsorbent for Cr (VI) and Pb (II) removal. J IRAN CHEM SOC (2024). https://doi.org/10.1007/s13738-024-03037-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13738-024-03037-3