Abstract
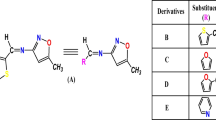
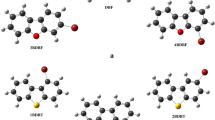
In order to investigate the effect of terminal donors on the structures and photophysical properties, four donor (D1)-acceptor (A)-π bridge-donor (D2) prototype fluorophores were synthesized and characterized. Single crystal analysis indicated that changing the types of terminal donors (D2) would give rise to different frontier molecular orbitals and molecular packing modes, leading to their different emission behaviors in solid states. In dilute solution, the four D–A–π-D compounds displayed different absorption and emission behaviors in dilute solution with solvatochromism properties. Assisted by the quantum chemical calculations and wave function analysis, the essence of optical properties of four compounds were explored. The results indicated that changing the types of terminal donors (D2) can affect the structures and photophysical properties of such donor (D1)-acceptor (A)-π bridge-donor (D2) prototype fluorophores, which provided guidance to design new fluorophores.










Similar content being viewed by others
References
J.H. Hong, S. Im, Y.J. Seo, N.Y. Kim, C.H. Ryu, M. Kim, K.M. Lee, Effect of terminal biphenyl ring geometry on the photophysical properties of close-o-carboranyl-anthracene dyads. J. Mater. Chem. C. 9(31), 9874–9883 (2021)
A. Nano, R. Ziessel, P. Stachelek, A. Harriman, Charge-Recombination fluorescence from push-pull electronic systems constructed around amino-substituted styryl-BODIPY dyes. Chem. Eur. J. 19(40), 13528–13537 (2013)
X. Zhang, Z. Chi, B. Xu, L. Jiang, X. Zhou, Y. Zhang, S. Liu, J. Xu, Multifunctional organic fluorescent materials derived from 9,10-distyrylanthracene with alkoxyl endgroups of various lengths. Chem. Commun. 48(88), 10895–10897 (2012)
M. Guerrini, A. Calzolari, S. Corni, Solid-state effects on the optical excitation of push-pull molecular J-aggregates by the first-principles simulations. ACS Omega 3(9), 10481–10486 (2018)
X. Wan, C. Li, M. Zhang, Y. Chen, Acceptor-donor-acceptor type molecules for high performance organic photovoltaics-chemistry and mechanism. Chem. Soc. Rev. 49(9), 2828–2842 (2020)
G. Yu, J. Gao, J.C. Hummelen, F. Wudl, A.J. Heeger, Polymer photovoltaic cells-enhanced efficiencies via a network of internal donor-acceptor heterojunctions. Science 270(5243), 1789–1792 (1995)
S. Günes, H. Neugebauer, N.S. Sariciftic, Conjugated polymer-based organic solar cells. Chem. Rev. 107(4), 1324–1338 (2007)
G. Dennler, M.C. Scharber, C.J. Brabec, Polymer-fullerene bulk-heterojunction solar cells. Adv. Mater. 21(13), 1323–1338 (2009)
F. Wurthner, Z.J. Chen, F.J.M. Hoeben, P. Osswald, C.C. You, P. Jonkheijm, J. von Herrikhuyzen, A. Schenning, P. van der Schoot, E.W. Meijer, E.H.A. Beckers, S.C.J. Meskers, R.A.J. Janssen, Supramolecular p-n-heterojunctions by co-self-organization of oligo(p-phenylene vinylene) and perylene bisimide dyes. J. Am. Chem. Soc. 126(34), 10611–10618 (2004)
E.H.A. Beckers, S.C.J. Meskers, A. Schenning, Z.J. Chen, F. Wurthner, P. Marsal, D. Beljonne, J. Cornil, R.A.J. Janssen, Influence of intermolecular orientation on the photoinduced charge transfer kinetics in self-assembled aggregates of donor-acceptor arrays. J. Am. Chem. Soc. 128(2), 649–657 (2006)
P. Kaur, M. Kaur, G. Depotter, S. van Cleuvenbergen, I. Asselberghs, K. Singh, Thermally stable ferrocenyl “push-pull” chromophores with tailorable swithchable second-order non-linear optical response: synthesis and structure-property relationship. J. Mater. Chem. 22(21), 10597–10608 (2012)
J.A. Mata, E. Peris, R. Llusar, S. Uriel, M.P. Cifuentes, M.G. Humphrey, M. Samoc, B. Luther-Davies, Synthesis, structures and nonlinear optical properties of ferrocenyl complexes with arylethenyl substituents. Eur. J. Inorg. Chem. 8, 2113–2122 (2001)
S. Goto, Y. Nitta, N.O. Decari, L.E. de Sousa, P. Stachelek, N. Tohnai, S. Minakata, P. de Silva, P. Data, Y. Takeda, Revealing the internal heavy chalcogen atom effect on the photophysics of the dibenzo[a, j]phenazine-cored donor-acceptor-donor triad. J. Mater. Chem. C. 9(39), 13942–13953 (2021)
A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo, H. Pettersson, Dye-sensitized solar cells. Chem. Rev. 110(11), 6595–6663 (2010)
T. Subash, R. Sen, P. Johari, Rationally designed donor-acceptor scheme based molecules for applications in opto-electronic devices. Phys. Chem. Chem. Phys. 18(13), 9133–9147 (2016)
D. Patra, T.Y. Huang, C.C. Chiang, R.O.V. Maturana, C.W. Pao, K.C. Ho, K.H. Wei, C.W. Chu, 2-Alkyl-5-thienyl-substituted benzo[1, 2-b: 4, 5-b’]dithiophene-based donor molecules for solution-processed organic solar cells. ACS Appl. Mater. Interfaces 5(19), 9494–9500 (2013)
D. Deng, Y. Zhang, L. Yuan, C. He, K. Lu, Z. Wei, Effects of shortened alkyl chains on solution-processable small molecules with oxo-alkylated nitrile end-capped acceptors for high-performance organic solar cells. Adv. Energy Mater. 4(17), 1400538 (2014)
Y. Wu, W. Zhu, Organic sensitizers from D-π-A to D-A-π-A: effect of the internal electron-withdrawing units on molecular sbsorption, energy levels and photovoltaic performances. Chem. Soc. Rev. 42(5), 2039–2058 (2013)
D.E. Wu, Q.H. Guo, Q. Qiao, Y.J. Cai, Q.Q. Zhou, The isomeric effect on the D-π-π-A prototype fluorescent materials: synthesis, photophysical property, and computation. Monatshefte für Chem-Chem. Mon. 152(11), 1315–1326 (2021)
D.E. Wu, Q.H. Guo, Evaluating the isomeric effects of donors on the structures and photophysical properties of donor-acceptor-p bridge-donor (D1-A-p-D2) prototype fluorophores. New J. Chem. 47(28), 13409–13421 (2023)
Q. Zhao, H. Lai, H. Chen, H. Li, F. He, H- and J-aggregation inspiring efficient solar conversion. J. Mater. Chem. A. 9(2), 1119–1126 (2021)
V. Kumar, G.A. Baker, S. Pandey, Ionic liquid-controlled J- versus H-aggregation of cyanine dyes. Chem. Commun. 47(16), 4730–4732 (2011)
J. Gierschner, S.Y. Park, Luminescent distyrylbenzenes: tailoring molecular structure and crystalline morphology. J. Mater. Chem. C. 1(37), 5818–5832 (2013)
A.B. Koren, M.D. Curtis, A.H. Francis, J.W. Kampf, Intermolecular interactions in π-stacked conjugated molecules: synthesis, structure, and spectral characterization of alkyl bithiazole oligomers. J. Am. Chem. Soc. 125(17), 5040–5050 (2003)
Z. Zhang, Y. Zhang, D. Yao, H. Bi, I. Javed, Y. Fan, H. Zhang, Y. Wang, Anthracene-arrangement-dependent emissions of crystals of 9-anthrylpyrazole derivatives. Cryst. Growth Des. 9(12), 5069–5076 (2009)
S. Kohmoto, R. Tsuyuki, H. Masu, I. Azumaya, K. Kishikawa, Polymorphism-dependent fluorescence of 9,10-bis(pentafluorobenzoyloxy)anthracene. Tetrahedron Lett. 49(1), 39–43 (2008)
T. Karatsu, T. Shibata, A. Nishigaki, A. Kitamura, Y. Hatanaka, Y. Nishimura, S.I. Sato, I. Yamazaki, π-π and σ-π interactions in α, ω-di-(9-anthryl) and di-(1-naphthyl)oligosilanes studied by time-resolved fluorescence in solution. J. Phys. Chem. B 107(44), 12184–12191 (2003)
Z.Y. Liu, T. Lu, Q. Chen, An sp-hybridized all-carboatmoic ring, cyclo[18]carbon: electronic structure, electronic spectrum, and optical nonlinearity. Carbon 165, 461–467 (2020)
T. Lu, Multiwfn Manual, Version 3.8 (dev), section 3.2.18, Accessed 30 October 2020. Aavailable at: http://sobereva.com/multiwfn/
H. Naito, K. Nishino, Y. Morisaki, K. Tanaka, Y. Chujo, Solid-state emission of the anthracene-o-carborane dyad form the twisted intramolecular charge transfer in solid state. Angew. Chem. Int. Ed. 56(1), 254–259 (2017)
K. Nishino, H. Yamamoto, K. Tanaka, Y. Chujo, Solid-state thermochromic luminescence through twisted intramolecular charge transfer and excimer formation of a carborane-pyrene dyad with an ethynyl spacer. Asian. J. Org. Chem. 6(12), 1818–1822 (2017)
A.V. Marsh, N.J. Cheetham, M. Little, M. Dyson, A.J.P. White, P. Beavis, C.N. Warriner, A.C. Swain, P.N. Stavrinou, M. Heeney, Carborane-induced excimer emission of severely twisted bis-o-carboranyl chrysene. Angew. Chem. Int. Ed. 57(33), 10640–10645 (2018)
D. Tahaoğlu, H. Usta, F. Alkan, Revisiting the role of charge transfer in the emission properties of carborane-fluorophore system: a TD-DFT investigation. J. Phys. Chem. A 126(26), 4199–4210 (2022)
S. Sasaki, Y. Niko, A.S. Klymchenko, G.I. Konishi, Design of donor-acceptor geomentry for tuning excited-state polarization: fluorescence solvatochromism of push-pull biphenyls with various torsional restrictions on their aryl-aryl bonds. Tetrahedron 70(41), 2731–2743 (2014)
L.O. Pålsson, C. Wang, A.S. Batsanov, S.M. King, A. Beeby, A.P. Monkman, M.R. Bryce, Efficient intramolecular charge transfer in oligoyne-linked donor-π-acceptor molecules. Chem. Eur. J. 16(5), 1470–1479 (2010)
F.L. Arbeloa, J.B. Prieto, V.M. Martínez, T.A. López, I.L. Arbeloa, Intramolecular charge transfer in pyrromethene laser dyes: photophysical behaviour of PM650. ChemPhysChem 5(11), 1762–1771 (2004)
L. Gobbi, N. Elmaci, H.P. Lüthi, F. Diederich, N, N-dialkylaniline-substituted tetraethynylethenes: a new class of chromophores possessing an emitting charge-transfer: experimental and computational studies. ChemPhysChem 2(1), 423–433 (2001)
Z.Y. Liu, X. Wang, T. Lu, A.H. Yuan, X.F. Yan, Potential optical molecular switch: Lithium@cyclo[18]carbon complex transforming between two stable configurations. Carbon 187, 78–85 (2022)
G. Haberhauer, R. Gleiter, C. Burkhart, Planarized intramolecular charge transfer: a concept for fluorophores with both large stokes shifts and high fluorescence quantum yields. Chem. Eur. J. 22(3), 971–978 (2016)
G. Haberhauer, Twisting of alkynes towards a carbon double helix. Chem. Eur. J. 23(50), 9288–9296 (2017)
Z.R. Grabowski, K. Rotkiewicz, W. Rettig, Structural changes accompanying intramolecular electron transfer: focus on twisted intramolecular charge-transfer states and structures. Chem. Rev. 103(10), 3899–4032 (2003)
G.M. Sheldrick, SHELXT-integrated space-group and crystal-structure determination. Acta Crystallogr. 71, 3–8 (2015)
Brandenburg K, DIAMOND, Version 3.1b, Crystal and Molecular Structure Visualization, Crystal Impact GbR, Bonn, Germany. 2006
Mercury 2.3 Supplied with Cambridge Structure Database. CCDC, Cambridge, UK 2003
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheesseman et al., Gaussian 16, Revision A.03 (Gaussian. Inc., Wallingford, 2016)
T. Lu, F.W. Chen, Multiwfn: a multifunctional wavefunctional analyzer. J. Comput. Chem. 33(5), 580–592 (2012)
W. Humphrey, A. Dalke, K. Schulten, VMD: visual molecular dynamics. J. Mol. Graph. 14(1), 33–38 (1996)
J.D. Chai, M. Head-Gordon, Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 10(44), 6615–6620 (2008)
F. Weigend, R. Ahlrichs, Balanced basis sets of split valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7(18), 3297–3305 (2005)
V. Barone, M. Cossi, Qantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 102(11), 1995–2001 (1998)
Acknowledgements
This work has been supported by the Applied Basic Research Program of Changzhou City (CJ20230012)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, XL., Guo, QH., Wu, JH. et al. Effect of terminal donors on the structures and photophysical properties of D–A-π-D small fluorophores. J IRAN CHEM SOC (2024). https://doi.org/10.1007/s13738-024-03035-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13738-024-03035-5