Abstract
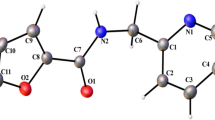
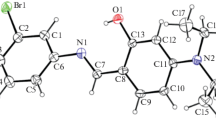
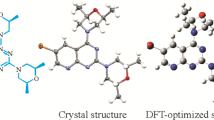
The synthesis of 1-(4(tert-butyl)-4-methoxy-[1,1-biphenyl]-4-yl) ethenone (4TBMBE) has been realized in excellent yield by using 4-tert-butyl phenylboronic acid and 1-bromonitrobenzene as the reactants. The single crystals were obtained by solvent-loss technique and its characterization was carried out using the spectral and X-ray diffraction methods. The molecule crystallizes in the monoclinic crystal system with space group (P21/c). There exists one C–H…O intramolecular and an intermolecular C–H···π interaction, besides one π···π (Cg···Cg) interaction responsible for stabilizing the unit cell molecular packing. The density functional theory has been employed to optimize the structure and to carry out the HOMO/LUMO analysis, computation of reactivity parameters, molecular electrostatic potential map and Mulliken population analysis. The Hirshfeld surface analysis probes the existence of various interactions in the crystal structure. Crystal voids analysis confirmed the absence of any significant cavity within the crystal packing. The relative contribution for each contact has been analyzed using the 2D fingerprint plots and their favourability has been validated by the enrichment ratio. The 3D topology of molecular packing has been visualized using energy framework analysis and the nature of molecular interactions existing within the structure has been ascertained using NCI-RDG analysis. The molecular docking investigations have been performed to study the anti-inflammatory action of 4TBMBE against the p38 MAP kinase inhibitor.













Similar content being viewed by others
References
M.P. Johansson, J. Olsen, J. Chem. Theory Comput. 4, 1460 (2008). https://doi.org/10.1021/ct800182e
G.P. Charbonneau, Y. Delugeard, Acta Crystallogr. B33, 1586 (1977). https://doi.org/10.1107/S0567740877006566
A. Almenningen, O. Bastiansen, L. Fernholt, B.N. Cyvin, S.J. Cyvin, S. Samdal, J. Mol. Struct. 128, 59 (1985). https://doi.org/10.1016/0022-2860(85)85041-9
V.J. Eaton, D. Steele, J. Chem. Soc. Faraday Trans. 69, 1601 (1973). https://doi.org/10.1039/f29736901601
Z.J. Jain, P.S. Gide, R.S. Kankate, Arab. J. Chem. 10, S2051 (2017). https://doi.org/10.1016/j.arabjc.2013.07.035
A.L.V. Kumar Reddy, N.E. Kathale, Orient. J. Chem. 33, 971 (2017). https://doi.org/10.13005/ojc/330250
J. Hassan, M. Sévignon, C. Gozzi, E. Schulz, M. Lemaire, Chem. Rev. 102, 1359 (2002). https://doi.org/10.1021/cr000664r
S. Ghasemi, S. Sharifi, J. Shahbazi Mojarrad, Adv. Pharm. Bull. 10, 423 (2020). https://doi.org/10.34172/apb.2020.051
R. Cincinelli, V. Zwick, L. Musso, V. Zuco, M. De Cesare, F. Zunino, C. Simoes-Pires, A. Nurisso, G. Giannini, M. Cuendet, S. Dallavalle, Eur. J. Med. Chem. 112, 99 (2016). https://doi.org/10.1016/j.ejmech.2016.02.001
C. Lee, H. Choi, E. Park, T. Nguyen, H. Maeng, K. Mee Lee, H. Jun, D. Shin, Biol. Drug Des. 98, 733 (2021). https://doi.org/10.1111/cbdd.13928
M. Pisano, M.A. Dettori, D. Fabbri, G. Delogu, G. Palmieri, C. Rozzo, Int. J. Mol. Sci. 22, 5636 (2021). https://doi.org/10.3390/ijms22115636
D. Zhao, S. Zhao, L. Zhao, X. Zhang, P. Wei, C. Liu, C. Hao, B. Sun, X. Su, M. Cheng, Bioorg. Med. Chem. 25, 750 (2017). https://doi.org/10.1016/j.bmc.2016.11.051
X. Wang, H.-Y. Fu, W. He, Y.-T. Xiang, Z.-C. Yang, Y. Kuang, S.-X. Yang, Curr. Issues Mol. Biol. 44, 4087 (2022). https://doi.org/10.3390/cimb44090280
N. Kumari, R. Sharma, A.A. Yadav, S.A. Sankpal, J.M. Raj, S. Murugavel, R. Kant, Eur. J. Chem. 14, 90 (2023). https://doi.org/10.5155/eurjchem.14.1.90-98.2386
N. Kumari, R. Sharma, A.A. Yadav, S.A. Sankpal, J.M. Raj, S. Murugavel, R. Kant, RASAYAN J. Chem. 16, 996 (2023). https://doi.org/10.31788/RJC.2023.1628136
N. Kumari, R. Sharma, A.A. Yadav, S.A. Sankpal, S. Murugavel, D. Lakshmanan, R. Kant, Ind. J. Pure Appl. Phys. (2023). https://doi.org/10.56042/ijpap.v61i9.3099
D. Rajnikant, G. Watkin, Tranter, Acta Crystallogr. C51, 2161 (1995). https://doi.org/10.1107/S0108270195005002
D.J. Rajnikant, G. Watkin, Tranter, Acta Crystallogr. C51, 2388–2390 (1995). https://doi.org/10.1107/S0108270195006329
D. Rajnikant, D. Singh, Bull. Mat. Sci. 27, 31 (2004). https://doi.org/10.1007/BF02708481
H. Kargar, M. Fallah-Mehrjardi, R. Behjatmanesh-Ardakani, M. Bahadori, M. Moghadam, M. Ashfaq, K.S. Munawar, M.N. Tahir, Inorg. Chem. Commun. 142, 109697 (2022). https://doi.org/10.1016/j.inoche.2022.109697
H. Kargar, M. Fallah-Mehrjardi, R. Behjatmanesh-Ardakani, M. Bahadori, M. Moghadam, M. Ashfaq, K.S. Munawar, M.N. Tahir, J. Coord. Chem. 75, 972 (2022). https://doi.org/10.1080/00958972.2022.2092846
H. Kargar, M. Fallah-Mehrjardi, R. Behjatmanesh-Ardakani, M. Bahadori, M. Moghadam, J. Javid, K.S. Munawar, J. Iran. Chem. Soc. 19, 3981 (2022). https://doi.org/10.1007/s13738-022-02583-y
R.M. Angell, P. Bamborough, A. Cleasby, S.G. Cockerill, K.L. Jones, C.J. Mooney, D.O. Somers, A.L. Walker, Bioorg. Med. Chem. Lett. 18, 318 (2008). https://doi.org/10.1016/j.bmcl.2007.10.076
G.M. Sheldrick, Acta Crystallogr. A64, 112 (2008). https://doi.org/10.1107/S0108767307043930
G.M. Sheldrick, Acta Crystallogr. A71, 3 (2015). https://doi.org/10.1107/S2053273314026370
O.V. Dolomanov, L.J. Bourhis, R.J. Gildea, J.A.K. Howard, H. Puschmann, J. Appl. Crystallogr. 42, 339 (2009). https://doi.org/10.1107/S0021889808042726
A.L. Spek, Acta Crystallogr. Sect. D: Biol. Crystallogr. 65, 148 (2009). https://doi.org/10.1107/S090744490804362X
M. Nardelli, J. Appl. Crystallogr. 28, 659 (1995). https://doi.org/10.1107/S0021889895007138
C.F. Macrae, I.J. Bruno, J.A. Chisholm, P.R. Edgington, P. McCabe, E. Pidcock, L. Rodriguez-Monge, R. Taylor, J. van de Streek, P.A. Wood, J. Appl. Crystallogr. 41, 466 (2008). https://doi.org/10.1107/S0021889807067908
A. Frisch, Gaussian 09W Reference (Wallingford, USA, 2009), p.25
A.D. Becke, J. Chem. Phys. 98, 5648 (1993). https://doi.org/10.1063/1.464913
A.D. Becke, Phys. Rev. A (Coll Park) 38, 3098 (1988). https://doi.org/10.1103/PhysRevA.38.3098
C. Lee, W. Yang, R.G. Parr, Phys. Rev. B 37, 785 (1988). https://doi.org/10.1103/PhysRevB.37.785
B. Miehlich, A. Savin, H. Stoll, H. Preuss, Chem. Phys. Lett. 157, 200 (1989). https://doi.org/10.1016/0009-2614(89)87234-3
P.R. Spackman, M.J. Turner, J.J. McKinnon, S.K. Wolff, D.J. Grimwood, D. Jayatilaka, M.A. Spackman, J. Appl. Crystallogr. 54, 1006 (2021). https://doi.org/10.1107/S1600576721002910
T. Lu, F. Chen, J. Comput. Chem. 33, 580 (2012). https://doi.org/10.1002/jcc.22885
W. Humphrey, A. Dalke, K. Schulten, J. Mol. Graph. 14, 33 (1996). https://doi.org/10.1016/0263-7855(96)00018-5
G.M. Morris, R. Huey, W. Lindstrom, M.F. Sanner, R.K. Belew, D.S. Goodsell, A.J. Olson, J. Comput. Chem. 30, 2785 (2009). https://doi.org/10.1002/jcc.21256
BIOVIA discovery studio visualizer Software version. vol. 20, pp. 779 (2016).
D. Rajnikant, G. Watkin, Tranter, Acta Crystallogr. C 51, 2071 (1995). https://doi.org/10.1107/S0108270195003593
J.J. Novina, G. Vasuki, S. Kumar, K.R.J. Thomas, Acta Crystallogr. E68, o319 (2012). https://doi.org/10.1107/S1600536812000347
H. Kargar, M. Fallah-Mehrjardi, R. Behjatmanesh-Ardakani, M. Bahadori, M. Moghadam, M. Ashfaq, K.S. Munawar, M.N. Tahir, Polyhedron 213, 115622 (2022). https://doi.org/10.1016/j.poly.2021.115622
S. Joshi, S. Vyas, M. Duffel, S. Parkin, H.-J. Lehmler, Synthesis 2011, 1045 (2011). https://doi.org/10.1055/s-0030-1258454
H. Kargar, M. Nateghi-Jahromi, M. Fallah-Mehrjardi, R. Behjatmanesh-Ardakani, K.S. Munawar, S. Ali, M. Ashfaq, M.N. Tahir, J. Mol. Struct. 1249, 131645 (2022). https://doi.org/10.1016/j.molstruc.2021.131645
H. Kargar, M. Fallah-Mehrjardi, R. Behjatmanesh-Ardakani, H.A. Rudbari, A.A. Ardakani, S. Sedighi-Khavidak, K.S. Munawar, M. Ashfaq, M.N. Tahir, Inorganica Chim. Acta. 530, 120677 (2022). https://doi.org/10.1016/j.ica.2021.120677
R. Manne, T. Åberg, Chem. Phys. Lett. 7, 282 (1970). https://doi.org/10.1016/0009-2614(70)80309-8
P.K. Chattaraj, B. Maiti, U. Sarkar, J. Phys. Chem. A 107, 4973 (2003). https://doi.org/10.1021/jp034707u
P. Politzer, J.S. Murray, Theoretical Chemistry accounts: theory, computation, and modeling. Theor. Chim. Acta 108, 134 (2002). https://doi.org/10.1007/s00214-002-0363-9
M.M.C. Ferreira, E. Suto, J. Phys. Chem. 96, 8844 (1992). https://doi.org/10.1021/j100201a030
J.J. McKinnon, D. Jayatilaka, M.A. Spackman, Chem. Commun. (2007). https://doi.org/10.1039/b704980c
H. Kargar, M. Fallah-Mehrjardi, R. Behjatmanesh-Ardakani, K.S. Munawar, M. Ashfaq, M.N. Tahir, J. Mol. Struct. 1250, 131691 (2022). https://doi.org/10.1016/j.molstruc.2021.131691
H. Kargar, M. Fallah-Mehrjardi, R. Behjatmanesh-Ardakani, K.S. Munawar, M. Ashfaq, M.N. Tahir, Inorg. Chim. Acta 527, 120568 (2021). https://doi.org/10.1016/j.ica.2021.120568
S.K. Seth, Acta Crystallogr. E74, 600 (2018). https://doi.org/10.1107/S2056989018003857
C. Jelsch, K. Ejsmont, L. Huder, IUCrJ 1, 119 (2014). https://doi.org/10.1107/S2052252514003327
M.G. Babashkina, K. Robeyns, Y. Filinchuk, D.A. Safin, New J. Chem. 40, 1230 (2016). https://doi.org/10.1039/C5NJ02588E
H. Kargar, M. Ashfaq, M. Fallah-Mehrjardi, R. Behjatmanesh-Ardakani, K.S. Munawar, M.N. Tahir, Inorg. Chim. Acta 536, 120878 (2022). https://doi.org/10.1016/j.ica.2022.120878
H. Amarne, W. Helal, D. Taher, M. Korb, A. Al-Hunaiti, Mol. Cryst. Liq. Cryst. 743, 77 (2022). https://doi.org/10.1080/15421406.2022.2050981
N.R. Sreenatha, A.S. Jeevan Chakravarthy, B. Suchithra, B.N. Lakshminarayana, S. Hariprasad, D.P. Ganesha, J. Mol. Struct. 1210, 127979 (2020). https://doi.org/10.1016/j.molstruc.2020.127979
C.D. Vincy, J.D.D. Tarika, X.D.D. Dexlin, A. Rathika, T.J. Beaula, J. Mol. Struct. 1247, 131388 (2022). https://doi.org/10.1016/j.molstruc.2021.131388
P. Akhileshwari, K.R. Kiran, M.A. Sridhar, M.P. Sadashiva, J. Mol. Struct. 1253, 132271 (2022). https://doi.org/10.1016/j.molstruc.2021.132271
A. Jamshidvand, M. Sahihi, V. Mirkhani, M. Moghadam, I. Mohammadpoor-Baltork, S. Tangestaninejad, H. Amiri Rudbari, H. Kargar, R. Keshavarzi, S. Gharaghani, J. Mol. Liq. 253, 61 (2018). https://doi.org/10.1016/j.molliq.2018.01.029
H. Kargar, R. Behjatmanesh-Ardakani, V. Torabi, M. Kashani, Z. Chavoshpour-Natanzi, Z. Kazemi, V. Mirkhani, A. Sahraei, M.N. Tahir, M. Ashfaq, K.S. Munawar, Polyhedron 195, 114988 (2021). https://doi.org/10.1016/j.poly.2020.114988
H. Kargar, R. Behjatmanesh-Ardakani, V. Torabi, A. Sarvian, Z. Kazemi, Z. Chavoshpour-Natanzi, V. Mirkhani, A. Sahraei, M. Nawaz Tahir, M. Ashfaq, Inorg. Chim. Acta. 514, 120004 (2021). https://doi.org/10.1016/j.ica.2020.120004
Acknowledgements
Neha Kumari is thankful to the UGC for granting NFSC under the Ministry of Social Justice and Empowerment, Government of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest in regard to this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumari, N., Yadav, A.A., Sankpal, S.A. et al. Synthesis, crystal structure, quantum chemical computation and molecular docking analysis of 1-(4(tert-butyl)-4-methoxy-[1,1-biphenyl]-4-yl) ethenone. J IRAN CHEM SOC (2024). https://doi.org/10.1007/s13738-024-03032-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13738-024-03032-8