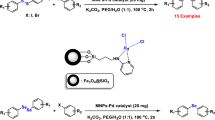
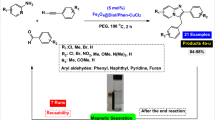
Abstract
Research on the preparation of diallyl sulfides and selenides is always an important challenge among chemists because these compounds are of high biological, pharmaceutical, industrial and chemical importance. For this purpose, in this attractive and highly efficient approach, we wish to report that copper (I) chloride immobilized on magnetic nanoparticles modified with benzothiazole–pyrimidine ligand (Fe3O4@BTH-Pyr-CuCl) is a novel and efficient magnetically recoverable catalyst for C–S and C–Se bonds formation through reaction of a category of heterocyclic compounds with aryl iodides, sulfur and selenium sources. The structure of Fe3O4@BTH-Pyr-CuCl nanocatalyst was well identified with FT-IR, SEM, TEM, EDX, elemental mapping, TGA, XRD, VSM and ICP-OES techniques. The recycling tests confirmed that the Fe3O4@BTH-Pyr-CuCl nanocatalyst was reused for 6 times without considerable reduction in its activity. This method is almost better than other methods reported in the literature for C–S and C–Se coupling of heterocycles for the following reasons, such as the use of an environmentally friendly solvent, high yields of products, the use of a catalyst that can be separated and reused and the performance of the reaction in a shorter time, presentation of well-analysis for catalyst and full NMR for products.
Graphical abstract








Similar content being viewed by others
References
A.K. Sharma, H. Joshi, A.K. Singh, RSC Adv. 10, 6452 (2020)
Y. Zhang, N. Song, Biol. Mol. Chem. 1, 53 (2023)
V.G. Pandya, S.B. Mhaske, Org. Lett. 16, 3836 (2014)
R. Chawla, L.D.S. Yadav, Org. Biomol. Chem. 17, 4761 (2019)
S. Gupta, J. Synth. Chem. 1, 16 (2022)
M. Kazemi, Nanomater. Chem. 1, 1 (2023)
S. Vajar, M. Mokhtary, Polycycl. Aromat. Compd. 39, 111 (2019)
E. Doustkhah, S. Rostamnia, M. Imura, Y. Ide, S. Mohammadi, C.J.T. Hyland, J. You, N. Tsunoji, B. Zeynizadeh, Y. Yamauchi, RSC Adv. 7, 56306 (2017)
R. Deilam, F. Moeinpour, F.S. Mohseni-Shahri, Monatshefte Für Chemie - Chem. Mon. 151, 1153 (2020)
M. Ghobadi, J. Synth. Chem. 1, 84 (2022)
M. Ghobadi, M. Kargar Razi, R. Javahershenas, M. Kazemi, Synth. Commun. 51, 647 (2021)
S. Rostamnia, E. Doustkhah, R. Bulgar, B. Zeynizadeh, Microporous Mesoporous Mater. 225, 272 (2016)
S. Rostamnia, K. Lamei, F. Pourhassan, RSC Adv. 4, 59626 (2014)
P. Ghamari Kargar, C. Len, R. Luque, Sustain. Chem. Pharm. 27, 100672 (2022)
R. Arundhathi, D. Damodara, P.R. Likhar, M.L. Kantam, P. Saravanan, T. Magdaleno, S.H. Kwon, Adv. Synth. Catal. 353, 1591 (2011)
L.S. Ardakani, A. Arabmarkadeh, M. Kazemi, Synth. Commun. 51(6), 856–879 (2021)
R. Taghavi, S. Rostamnia, M. Farajzadeh, H. Karimi-Maleh, J. Wang, D. Kim, H.W. Jang, R. Luque, R.S. Varma, M. Shokouhimehr, Inorg. Chem. 61, 15747 (2022)
F.M. Moghaddam, M. Eslami, Appl. Organomet. Chem. 32, e4463 (2018)
M.R. Abdi, Biol. Mol. Chem. 1, 1 (2023)
A. Baghban, M. Heidarizadeh, E. Doustkhah, S. Rostamnia, P.F. Rezaei, Int. J. Biol. Macromol. 103, 1194 (2017)
A.R. Sardarian, F. Mohammadi, M. Esmaeilpour, Res. Chem. Intermed. 45, 1437 (2019)
E. Doustkhah, M. Heidarizadeh, S. Rostamnia, A. Hassankhani, B. Kazemi, X. Liu, Mater. Lett. 216, 139 (2018)
R. Eisavi, A. Karimi, RSC Adv. 9, 29873 (2019)
M. Kazemi, Synth. Commun. 50, 1899 (2020)
J. Hou, M. Kazemi, Res. Chem. Intermed. 50, 1845–1872 (2024)
A. Noory Fajer, H. Khabt Aboud, H.A. Al-Bahrani, M. Kazemi, Polycycl. Aromat. Compd. 43, 1–47 (2023). https://doi.org/10.1080/10406638.2023.2255723
M. Lakshman, J. Synth. Chem. 1, 48 (2022)
S. Sajjadifar, M.A. Zolfigol, F. Tami, J. Chinese Chem. Soc. 66, 307 (2019)
K. Takagi, Chem. Lett. 16, 2221 (1987)
L. Shiri, A. Ghorbani-Choghamarani, M. Kazemi, Aust. J. Chem. 69, 585 (2016)
M. Kazemi, Synth. Commun. 50, (2020).
I.M. Yonova, C.A. Osborne, N.S. Morrissette, E.R. Jarvo, J. Org. Chem. 79, 1947 (2014)
P. Anbarasan, H. Neumann, M. Beller, Chem. Commun. 47, 3233 (2011)
X. Li, T. Yuan, Y. Yang, J. Chen, Tetrahedron 70, 9652 (2014)
L.-F. Niu, Y. Cai, C. Liang, X.-P. Hui, P.-F. Xu, Tetrahedron 67, 2878 (2011)
R. Zhang, H. Ding, X. Pu, Z. Qian, Y. Xiao, Catalysts 10, 1339 (2020)
M. Vaddamanu, K. Velappan, G. Prabusankar, New J. Chem. 44, 129 (2020)
M. Arisawa, T. Ichikawa, M. Yamaguchi, Org. Lett. 14, 5318 (2012)
C.C. Eichman, J.P. Stambuli, Molecules 16, 590 (2011)
X. Xu, W. Wang, L. Lu, J. Zhang, J. Luo, Catal. Letters 152, 3031 (2022)
V. Rathore, S. Kumar, Green Chem. 21, 2670 (2019)
Y. Kobiki, S. Kawaguchi, T. Ohe, A. Ogawa, Beilstein J. Org. Chem. 9, 1141 (2013)
A.R. Rosario, K.K. Casola, C.E.S. Oliveira, G. Zeni, Adv. Synth. Catal. 355, 2960 (2013)
G. Kumaraswamy, V. Ramesh, M. Gangadhar, S. Vijaykumar, Asian J. Org. Chem. 7, 1689 (2018)
H. Chuai, S.-Q. Zhang, H. Bai, J. Li, Y. Wang, J. Sun, E. Wen, J. Zhang, M. Xin, Eur. J. Med. Chem. 223, 113621 (2021)
D. Hu, M. Liu, H. Wu, W. Gao, G. Wu, Org. Chem. Front. 5, 1352 (2018)
X. Liu, S.-B. Zhang, H. Zhu, Z.-B. Dong, J. Org. Chem. 83, 11703 (2018)
L. Chen, A. Noory Fajer, Z. Yessimbekov, M. Kazemi, M. Mohammadi, J. Sulfur Chem. 40, 451 (2019)
M.M. Khodaei, A. Alizadeh, M. Haghipour, Res. Chem. Intermed. 45, 2727 (2019)
G. Balakishan, G. Kumaraswamy, V. Narayanarao, P. Shankaraiah, Heterocycl. Commun. 27, 17 (2021)
D. Kumar, B.B. Mishra, V.K. Tiwari, J. Org. Chem. 79, 251 (2014)
X. Hao, D. Feng, H. Chen, P. Huang, F. Guo, Chem.—A Eur. J. 29(60), e202302119 (2023)
V.N. Bochatay, P.J. Boissarie, J.A. Murphy, C.J. Suckling, S. Lang, J. Org. Chem. 78, 1471 (2013)
S. Ranjit, R. Lee, D. Heryadi, C. Shen, J. Wu, P. Zhang, K.-W. Huang, X. Liu, J. Org. Chem. 76, 8999 (2011)
P. Gandeepan, J. Mo, L. Ackermann, Chem. Commun. 53, 5906 (2017)
J. Rafique, G. Farias, S. Saba, E. Zapp, I.C. Bellettini, C.A. Momoli Salla, I.H. Bechtold, M.R. Scheide, J.S. Santos Neto, D. Monteiro de Souza Junior, H. de Campos Braga, L.F.B. Ribeiro, F. Gastaldon, C.T. Pich, T.E.A. Frizon, Dye. Pigment. 180, 108519 (2020)
R. Wang, H. Xu, Y. Zhang, Y. Hu, Y. Wei, X. Du, H. Zhao, Org. Biomol. Chem. 19, 5899 (2021)
X. Ren, Q. Liu, Z. Yang, Z. Wang, X. Chen, Chinese Chem. Lett. 34, 107821 (2023)
C. Ravi, D. Chandra Mohan, S. Adimurthy, Org. Biomol. Chem. 14, 2282 (2016)
S. Kundu, B. Basu, RSC Adv. 5, 50178 (2015)
Y. An, G. Xu, M. Cai, S. Wang, X. Zhong Wang, Y. Chen, L. Dai, Tetrahedron 79, 131829 (2021)
Y.-S. Zhu, Y. Xue, W. Liu, X. Zhu, X.-Q. Hao, M.-P. Song, J. Org. Chem. 85, 9106 (2020)
C. Gao, G. Wu, L. Min, M. Liu, W. Gao, J. Ding, J. Chen, X. Huang, H. Wu, J. Org. Chem. 82, 250 (2017)
J.M. Anghinoni, S.S. Ferreira, R.F. Schumacher, B.A. Iglesias, G. Perin, F. Penteado, E.J. Lenardão, New J. Chem. 47, 6066 (2023)
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, K., Chang, LY. Copper complex supported on the surface of magnetic nanoparticles: an ecofriendly catalyst for C–S and C–Se coupling reactions. J IRAN CHEM SOC (2024). https://doi.org/10.1007/s13738-024-03015-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13738-024-03015-9