Abstract
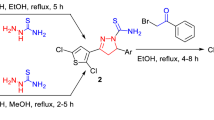
A facile one pot multi-component synthesis of novel 1,3,4-thiadiazine and thiazole derivatives from the reaction of (methyl-1-(p-tolyl)-1H-1,2,3-triazol-4-yl)ethan-1-one, thiocarbohydrazide/or thiosemicarbazide, and aromatic aldehydes. The synthesized compounds were evaluated for antimicrobial, antibiofilm, and antioxidant activity. The results revealed the marked potency as antimicrobial agents. Thiazole 6e has the highest antimicrobial activity. Furthermore, most compounds inhibited biofilms produced by Pseudomonas aeruginosa. Promising thiazole 6c and thiadiazine derivatives (4c and 4 g) exhibited superior antioxidant activity and remarkable scavenging activity with DPPH, (86.99 ± 1.1, 81.54 ± 3.4 and 78.25 ± 1.8) and ABTS (80.06 ± 0.12, 62.29 ± 0.31 and 44.38 ± 0.20) respectively. The molecular docking simulation showed lower binding energy with different types of interaction at the active site of Dihydropteroate synthase, Sortase A, LasR, and Penicillin-binding proteins pockets indicating that these compounds could inhibit the enzyme and cause promising antimicrobial effects. Also, Molecular docking to the active sites of Peroxidase enzyme as an antioxidant receptor revealed that thiadiazine derivatives (4c and 4d) displayed minimal binding energy and have a good affinity toward the active pocket of each enzyme.
Graphical Abstract











Similar content being viewed by others
References
X.-P. Hui, H.-S. Dong, P.-F. Xu, Z.-Y. Zhang, Q. Wang, Y.-N. Gong, J. Chin. Chem. Soc. 47, 1115–1119 (2000)
S. Kumari, S.K. Gupta, L. Tiwari, Int. J. Pharm. Erud. 11(2), 22–48 (2021)
D. Gupta, D.K.J. Jain, J. Adv. Pharm. Technol. Res. 6, 141–146 (2015)
R. Kharb, M.S. Yar, P.C. Sharma, Mini Rev. Med. Chem. 11, 84–96 (2011)
R. Paprocka, M. Wiese, A. Eljaszewicz, A. Helmin-Basa, A. Gzella, B. Modzelewska-Banachiewicz, J. Michalkiewicz, Bioorg. Med. Chem. Lett. 25, 2664–2667 (2015)
N. Pokhodylo, O. Shyyka, V. Matiychuk, Sci. Pharm. 81, 663–676 (2013)
A.A. Abd-Rabou, B.F. Abdel-Wahab, M.S. Bekheit, Chem. Pap. 72(9), 2225–2237 (2018)
Z.A. Kaplancıklı, G. Turan-Zitouni, A. Özdemir, G. Revial, Eur. J. Med. Chem. 43, 155–159 (2008)
E. Fotopoulou, M. Fragkiadakis, C.G. Neochoritis, Tetrahedron Lett. 120, 1544–1553 (2023)
A. Das, M.W. Ashraf, B.K. Banik, ChemistrySelect 6(34), 9069–9100 (2021)
B.N. Goswami, J.C.S. Kataky, J.N. Baruah, J. Heterocycl. Chem. 23, 1439–1442 (1986)
S.S.P. Garoufalias, O.G. Todoulou, E.C. Filippatos, A.E.P. Valiraki, A. Chytirogiou-Lada, Arzneim., Forsch Drug Res. 48, 1019–1023 (1998)
Z. Sui, J. Guan, D.J. Hlasta, M.J. Macielag, B.D. Foleno, R.M. Goldschmidt, M.J. Loeloff, G.C. Webb, J.F. Barrett, Bioorg. Med. Chem. Lett. 8, 1929–1934 (1998)
M.M. Ibrahim, H. Abumahmoud, A.T. Al-Fawwaz, J. Iran. Chem. Soc. 19, 2811–2822 (2022). https://doi.org/10.1007/s13738-022-02495-x
M. Jyothi, H.A. Khamees, S.M. Patil et al., J. Iran. Chem. Soc. 19, 3919–3933 (2022). https://doi.org/10.1007/s13738-022-02574-z
T. El Malah, H.F. Nour, A.A.E. Satti, B.A. Hemdan, W.A. El-Sayed, Molecules 25(4), 790 (2020)
H.R.M. Rashdan, I.A. Shehadi, M.T. Abdelrahman, B.A. Hemdan, Molecules 26(16), 4817 (2021)
I.H. El Azab, H.S. El-Sheshtawy, R.B. Bakr, N.A.A. Elkanzi, Molecules 26(3), 708 (2021)
S. Punia, V. Verma, D. Kumar, A. Kumar, L. Deswal, G. Singh, S.C. Sahoo, J. Mol. Struct. 1262, 133060 (2022)
C. Deng, H. Yan, J. Wang, K. Liu, B.-S. Liu, Y.-M. Shi, Eur. J. Med. Chem. 244, 114888 (2022)
L. Deswal, V. Verma, D. Kumar, Chem. Pap. 76, 7607–7622 (2022)
M.L. Rodrigues, ASM Journals/mBio 9(5), e01755-e1818 (2018)
A. Ganeshkumar, S. Suvaithenamudhan, R. Rajaram, Curr. Microbiol. 1–11 (2020).
S.H. Satuluri, S.K. Katari, C. Pasala, U. Amineni, J. Recept. Signal Transduction 40(3), 246–256 (2020)
C., Capasso, C. T., Bacterial Resist. Antibiotics–From Molecules to Man 163–172 (2019).
S.A. Cochrane, C.T. Lohans, Eur. J. Med. Chem. 194, 112262 (2020)
L. M. Lima, B. N. M. da Silva, G. Barbosa, E. J. Barreiro, Eur. J. Med. Chem. 112829 (2020).
L. Braun, S. Dramsi, P. Dehoux, H. Bierne, G. Lindahl, P. Cossart, Mol. Microbiol. 25(2), 285–294 (1997)
G.A. AsanteAmpadu, J.O. Mensah, G. Darko, L.S. Borquaye, Evid. Based Complem Alternat Med. (2022). https://doi.org/10.1155/2022/7211015
S. R. Elgogary, E. M. ElTelbani, R. E. Khidre, Polycyclic Aromat. Compd. (2022). https://doi.org/10.1080/10406638.2022.2140170
R.E. Khidre, I.A.M. Radini, Sci. Rep. 11, 7846 (2021)
H.A. Mohamed, R.E. Khidre, B.M. Kariuki, G.A. El-Hiti, J. Heterocycl. Chem. 57, 1055–1062 (2020)
R.E. Khidre, H.A. Mohamed, B.M. Kariuki, G.A. El-Hiti, Phosphorus Sulfur Silicon Relat. Elem. 195, 29–36 (2020)
S.R. Elgogary, R.E. Khidre, E.M. El-Telbani, J. Iran. Chem. Soc. 17, 765–776 (2020)
R.E. Khidre, I.A.M. Radini, J. Heterocycl. Chem. 56, 850–858 (2019)
G. Palla, C. Pelizzi, G. Predieri, C. Vignali, Gazz. Chim. Ital. 112, 339–341 (1982)
E. Wyrzykiewicz, A. Błaszczyk, I. Turowska-Tyrk, Bull. Pol. Acad. Sci. Chem. 48, 213–229 (2000)
V.V. Syakaev, S.N. Podyachev, B.I. Buzykin, S.K. Latypov, W.D. Habicher, A.I. Konovalov, J. Mol. Struct. 788, 55–62 (2006)
G. Palla, G. Predieri, P. Domiano, Tetrahedron 42, 3649–3654 (1986)
E. Wyrzykiewicz, D. Prukała, J. Heterocycl. Chem. 35, 381–387 (1998)
Z.-P. Cao, W.-J. Dong, H.-S. Dong, Indian. J. Heterocycl. Chem. 48B, 873–876 (2009)
R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, C. Rice-Evans, Free Radical Biol. Med. 26, 1231–1237 (1999)
L. L. Mensor, F. S. Menezes, G. G. Leitão, A. S. Reis, T. C. d. Santos, C. S. Coube, S. G. Leitão, Phytotherapy Res. 15,127–130 (2001).
S. Magaldi, S. Mata-Essayag, C.H. De Capriles, C. Pérez, M. Colella, C. Olaizola, Y. Ontiveros, Int. J. infect. Dis. 8, 39–45 (2004)
R. Hernandez-Delgadillo, D. Velasco-Arias, D. Diaz, K. Arevalo-Niño, M. Garza-Enriquez, M. A. De la Garza-Ramos, C. Cabral-Romero, Int. J. Nanomed., 2109–2113 (2012).
A. Daina, O. Michielin, V. Zoete, Sci. Rep. 7(1), 42717 (2017)
N.M. Boyle, M. Banck, C.A. James, C. Morley, T. Vandermeersch, G.R. Hutchison, J. Cheminform. 3(33), 1–14 (2011)
J. Eberhardt, D. Santos-Martins, A.F. Tillack, S. Forli, J. Chem. Inf. Model. 61(8), 3891–3898 (2021)
Author information
Authors and Affiliations
Contributions
REK: Conceptualization, Investigation, Supervision, Methodology, Writing – original draft. ES: Methodology, Investigation, Writing – review & editing. AFES: Methodology, Investigation, Writing – original draft. AAS: Methodology, Investigation, Writing – review & editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khidre, R.E., Sabry, E., El-Sayed, A.F. et al. Design, one-pot synthesis, in silico ADMET prediction and molecular docking of novel triazolyl thiadiazine and thiazole derivatives with evaluation of antimicrobial, antioxidant and antibiofilm inhibition activity. J IRAN CHEM SOC 20, 2923–2947 (2023). https://doi.org/10.1007/s13738-023-02889-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-023-02889-5