Abstract
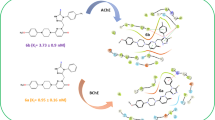
The bis(pyridine-2(1H)-thione) was prepared and taken as a key synthon of this study. The target bis(pyrazolo[3,4-b]pyridines) was prepared, in good yields, by the reaction of bis(pyridine-2(1H)-thione) with appropriate hydrazonyl chlorides to yield bis(hydrazonothioates) followed by their heating in ethanolic sodium ethoxide solution. Additionally, bis(pyridine-2(1H)-thione) reacted with different α-halogenated reagents to afford a new series of bis(thieno[2,3-b]pyridines), in good to excellent yields. In general, the tested series of bis(thieno[2,3-b]pyridines) demonstrated greater acetylcholinesterase inhibitory activity as well as DPPH antioxidant activity than the other series of (pyrazolo[3,4-b]pyridines). At a concentration of 100 μM, bis(thieno[2,3-b]pyridine-2-carbonitrile) showed the best acetylcholinesterase inhibitory activity with inhibition percentage of 83.2. In addition, the previous hybrid had the highest DPPH antioxidant activity, with an inhibition percentage of 82.6 when tested at a concentration of 25 μg/mL. Furthermore, SwissADME was used to predict the physicochemical properties, lipophilicity, and drug likeness of the new products.
Graphical abstract












Similar content being viewed by others
References
E.D. AlFadly, P.A. Elzahhar, A. Tramarin, S. Elkazaz, H. Shaltout, M.M. Abu-Serie, J. Janockova, O. Soukup, D.A. Ghareeb, A.F. El-Yazbi, R.W. Rafeh, Eur. J. Med. Chem. 167, 161–186 (2019). https://doi.org/10.1016/j.ejmech.2019.02.012
M. Saxena, R. Dubey, Curr. Top. Med. Chem. 19, 264–275 (2019). https://doi.org/10.2174/1568026619666190128125912
W. Liu, M. Lang, M.B. Youdim, T. Amit, Y. Sun, Z. Zhang, Y. Wang, O. Weinreb, Neuropharmacology 109, 376–385 (2016). https://doi.org/10.1016/j.neuropharm.2016.06.013
A. Kumar, A. Singh, Pharmacol. Rep. 67, 195–203 (2015). https://doi.org/10.1016/j.pharep.2014.09.004
A.S. Gurjar, M.N. Darekar, K.Y. Yeong, L. Ooi, Bioorg. Med. Chem. 26, 1511–1522 (2018). https://doi.org/10.1016/j.bmc.2018.01.029
J. Trujillo-Ferrara, L. Montoya Cano, M. Espinoza-Fonseca, Bioorg. Med. Chem. Lett. 13, 1825–1827 (2003). https://doi.org/10.1016/S0960-894X(03)00198-7
F. Vafadarnejad, M. Mahdavi, E. Karimpour-Razkenari, N. Edraki, B. Sameem, M. Khanavi, M. Saeedi, T. Akbarzadeh, Bioorg. Chem. 77, 311–319 (2018). https://doi.org/10.1016/j.bioorg.2018.01.013
J. Joubert, G.B. Foka, B.P. Repsold, D.W. Oliver, E. Kapp, S.F. Malan, Eur. J. Med. Chem. 125, 853–864 (2017). https://doi.org/10.1016/j.ejmech.2016.09.041
M.T. Heneka, M.J. Carson, J. El Khoury, G.E. Landreth, F. Brosseron, D.L. Feinstein, A.H. Jacobs, T. Wyss-Coray, J. Vitorica, R.M. Ransohoff, K. Herrup, Lancet Neurol. 14, 388–405 (2015). https://doi.org/10.1016/S1474-4422(15)70016-5
C.K. Glass, K. Saijo, B. Winner, M.C. Marchetto, F.H. Gage, Cell 140, 918–934 (2010). https://doi.org/10.1016/j.cell.2010.02.016
J. Hardy, J. Neurochem. 110, 1129–1134 (2009). https://doi.org/10.1111/j.1471-4159.2009.06181.x
M. Weinstock, E. Groner, Chem. Biol. Interact. 175, 216–221 (2008). https://doi.org/10.1016/j.cbi.2008.03.014
J.L. Cummings, R. Doody, C. Clark, Neurology 69, 1622–1634 (2007). https://doi.org/10.1212/01.wnl.0000295996.54210.69
J. Rodda, J. Carter, BMJ 344, e2986 (2012). https://doi.org/10.1136/bmj.e2986
A. Więckowska, M. Kołaczkowski, A. Bucki, J. Godyń, M. Marcinkowska, K. Więckowski, P. Zaręba, A. Siwek, G. Kazek, M. Głuch-Lutwin, P. Mierzejewski, Eur. J. Med. Chem. 124, 63–81 (2016). https://doi.org/10.1016/j.ejmech.2016.08.016
M.B. Colovic, D.Z. Krstic, T.D. Lazarevic-Pasti, A.M. Bondzic, V.M. Vasic, Curr. Neuropharmacol. 11, 315–335 (2013). https://doi.org/10.2174/1570159X11311030006
M. Mroueh, W.H. Faour, W.N. Shebaby, C.F. Daher, T.M. Ibrahim, H.M. Ragab, Bioorg. Chem. 100, 103895 (2020). https://doi.org/10.1016/j.bioorg.2020.103895
T. Umar, S. Shalini, M.K. Raza, S. Gusain, J. Kumar, P. Seth, M. Tiwari, N. Hoda, Eur. J. Med. Chem. 175, 2–19 (2019). https://doi.org/10.1016/j.ejmech.2019.04.038
M. do Carmo Carreiras, J. Marco-Contelles, Synlett 32, 1987–2012 (2021). https://doi.org/10.1055/s-0040-1719823
M. Saeedi, M. Safavi, E. Allahabadi, A. Rastegari, R. Hariri, S. Jafari, S.N. Bukhari, S.S. Mirfazli, O. Firuzi, N. Edraki, M. Mahdavi, Arch. Pharm. 353, 2000101 (2020). https://doi.org/10.1002/ardp.202000101
B. Zhao, Y. Li, P. Xu, Y. Dai, C. Luo, Y. Sun, J. Ai, M. Geng, W. Duan, A.C.S. Med, Chem. Lett. 7, 629–634 (2016). https://doi.org/10.1021/acsmedchemlett.6b00066
R.F. Barghash, W.M. Eldehna, M. Kovalová, V. Vojáčková, V. Kryštof, H.A. Abdel-Aziz, Eur. J. Med. Chem. 227, 113952 (2022). https://doi.org/10.1016/j.ejmech.2021.113952
P. Nagender, G.M. Reddy, R.N. Kumar, Y. Poornachandra, C.G. Kumar, B. Narsaiah, Bioorg. Med. Chem. Lett. 24, 2905–2908 (2014). https://doi.org/10.1016/j.bmcl.2014.04.084
M.A. El-Borai, H.F. Rizk, M.F. Abd-Aal, I.Y. El-Deeb, Eur. J. Med. Chem. 48, 92–96 (2012). https://doi.org/10.1016/j.ejmech.2011.11.038
A.M.R. Bernardino, A.R. de Azevedo, L.C. da Silva Pinheiro, J.C. Borges, V.L. Carvalho, M.D. Miranda, M.D.F. de Meneses, M. Nascimento, D. Ferreira, M.A. Rebello, V.A.G.G. Da Silva, Med. Chem. Res. 16, 352–369 (2007). https://doi.org/10.1007/s00044-007-9035-6
A.C. Medeiros, J.C. Borges, K.M. Becker, R.F. Rodrigues, L.L. Leon, M. Canto-Cavalheiro, A.M. Bernardino, M.C.D. Souza, L.F. Pedrosa, J. Braz. Chem. Soc. 29, 159–167 (2018). https://doi.org/10.21577/0103-5053.20170126
S.M.H. Sanad, A.E.M. Mekky, Chem. Biodivers. 19, e202100500 (2022). https://doi.org/10.1002/cbdv.202100500
I.H. Eissa, A.M. El-Naggar, M.A. El-Hashash, Bioorg. Chem. 67, 43–56 (2016). https://doi.org/10.1016/j.bioorg.2016.05.006
C.M.S. Menezes, C.M.R. Sant’Anna, C.R. Rodrigues, E.J. Barreiro, J. Mol. Struct. 579, 31–39 (2002). https://doi.org/10.1016/S0166-1280(01)00677-7
S.M. Emam, F.A. El-Saied, S.A. Abou El-Enein, H.A. El-Shater, Spectrochim. Acta Part A 72, 291–297 (2009). https://doi.org/10.1016/j.saa.2008.09.015
J. Quiroga, Y. Villarreal, J. Gálvez, A. Ortíz, B. Insuasty, R. Abonia, M. Raimondi, S. Zacchino, Chem. Pharm. Bull. 65, 143–150 (2017). https://doi.org/10.1248/cpb.c16-00652
P.K. Sharma, K. Singh, S. Kumar, P. Kumar, S.N. Dhawan, S. Lal, H. Ulbrich, G. Dannhardt, Med. Chem. Res. 20, 239–244 (2011). https://doi.org/10.1007/s00044-010-9312-7
S.M.H. Sanad, A.E.M. Mekky, J. Iran. Chem. Soc. 17, 3299–3315 (2020). https://doi.org/10.1007/s13738-020-01987-y
D. Shuck-Lee, F.F. Chen, R. Willard, S. Raman, R. Ptak, M.L. Hammarskjold, D. Rekosh, Antimicrob. Agents Chemother. 52, 3169–3179 (2008). https://doi.org/10.1128/AAC.00274-08
K. Madhusudana, B. Shireesha, V.G.M. Naidu, S. Ramakrishna, B. Narsaiah, A.R. Rao, P.V. Diwan, Eur. J. Pharmacol. 678, 48–54 (2012). https://doi.org/10.1016/j.ejphar.2011.12.019
M. Kamata, T. Yamashita, A. Kina, M. Funata, A. Mizukami, M. Sasaki, A. Tani, M. Funami, N. Amano, K. Fukatsu, Bioorg. Med. Chem. Lett. 22, 3643–3647 (2012). https://doi.org/10.1016/j.bmcl.2012.04.047
E.M. Mohi El-Deen, A. El-Meguid, A. Eman, S. Hasabelnaby, E.A. Karam, E.S. Nossier, Molecules 24, 3650–3669 (2019). https://doi.org/10.3390/molecules24203650
M.S. Mohamed, Y.E. Mansour, H.K. Amin, M.E. El-Araby, J. Enzyme Inhibit. Med. Chem. 33, 755–767 (2018). https://doi.org/10.1080/14756366.2018.1457657
C. Karthikeyan, R. Malla, C.R. Ashby Jr., H. Amawi, K.L. Abbott, J. Moore, J. Chen, C. Balch, C. Lee, P.C. Flannery, P. Trivedi, Cancer Lett. 376, 118–126 (2016). https://doi.org/10.1016/j.canlet.2016.03.030
S.M.H. Sanad, A.E.M. Mekky, ChemistrySelect 5, 8494–8503 (2020). https://doi.org/10.1002/slct.202001208
A. Daina, O. Michielin, V.A. Zoete, Sci. Rep. 7, 42717 (2017). https://doi.org/10.1038/srep42717
A.A.M. Ahmed, A.E.M. Mekky, S.M.H. Sanad, Synth. Commun. 52, 912–925 (2022). https://doi.org/10.1080/00397911.2022.2056853
A.E.M. Mekky, S.M.H. Sanad, T.T. El-Idreesy, Synth. Commun. 51, 3332–3344 (2021). https://doi.org/10.1080/00397911.2021.1970774
A.E.M. Mekky, S.M.H. Sanad, J. Heterocyclic Chem. 57, 4278–4290 (2020). https://doi.org/10.1002/jhet.4134
S.M.H. Sanad, A.E.M. Mekky, J. Iran. Chem. Soc. 18, 213–224 (2021). https://doi.org/10.1007/s13738-020-02018-6
A. Tlili, F. Monnier, M. Taillefer, Chem. Eur. J. 16, 12299–12302 (2010). https://doi.org/10.1002/chem.201001373
A.E.M. Mekky, A.S. Al-Bogami, J. Heterocycl. Chem. 53, 595–605 (2016). https://doi.org/10.1002/jhet.2328
A.S. Al-Bogami, A.E.M. Mekky, J. Heterocycl. Chem. 53, 1554–1562 (2016). https://doi.org/10.1002/jhet.2462
B. Hu, P. Zhou, Q. Zhang, Y. Wang, S. Zhao, L. Lu, S. Yan, F. Yu, J. Org, Chem. 83, 14978–14986 (2018). https://doi.org/10.1021/acs.joc.8b02235
Q. Chen, X. Wanga, C. Wena, Y. Huanga, X. Yana, J. Zeng, RSC Adv. 7, 39758–39761 (2017). https://doi.org/10.1039/C7RA06904A
A.S. Kulikov, M.A. Epishina, E.S. Zhilin, A.D. Shuvaev, L.L. Fershtat, N.N. Makhova, Mendeleev Commun. 31, 42–45 (2021). https://doi.org/10.1016/j.mencom.2021.01.012
A.E.M. Mekky, S.M.H. Sanad, Polycyclic Aromat. Compd. 41, 936–949 (2021). https://doi.org/10.1080/10406638.2019.1631194
C.M. Holden, M.F. Greaney, Chem. Eur. J. 23, 8992–9008 (2017). https://doi.org/10.1002/chem.201700353
S.M.H. Sanad, A.E.M. Mekky, J. Heterocycl. Chem. 57, 3142–3152 (2020). https://doi.org/10.1002/jhet.4021
S.M.H. Sanad, A.M. Abdel Fattah, F.A. Attaby, M.A.A. Elneairy, Can. J. Chem. 97, 53–60 (2019). https://doi.org/10.1139/cjc-2017-0721
G.L. Ellman, K.D. Courtney, V. Andres Jr., R.M. Featherstone, Biochem. Pharm. 7, 88–90 (1961). https://doi.org/10.1016/0006-2952(61)90145-9
M. Alipour, M. Khoobi, A. Foroumadi, H. Nadri, A. Moradi, A. Sakhteman, M. Ghandi, A. Shafiee, Bioorg. Med. Chem. 20, 7214–7222 (2020). https://doi.org/10.1016/j.bmc.2012.08.052
T. Pan, S. Xie, Y. Zhou, J. Hu, H. Luo, X. Li, L. Huang, Bioorg. Med. Chem. Lett. 29, 2150–2152 (2019). https://doi.org/10.1016/j.bmcl.2019.06.056
D. Silva, M. Chioua, A. Samadi, M.C. Carreiras, M.L. Jimeno, E. Mendes, C. de Los Ríos, A. Romero, M. Villarroya, M.G. López, J. Marco-Contelles, Eur. J. Med. Chem. 46, 4676–4681 (2011). https://doi.org/10.1016/j.ejmech.2011.05.068
E.J. Barreiro, C.A. Camara, H. Verli, L. Brazil-Más, N.G. Castro, W.M. Cintra, Y. Aracava, C.R. Rodrigues, C.A. Fraga, J. Med. Chem. 46, 1144–1152 (2003). https://doi.org/10.1021/jm020391n
F.A. Attaby, A.M. Abdel-Fattah, L.M. Shaif, M.M. Elsayed, Phosphorus Sulfur Silicon Relat. Elem. 185, 129–139 (2009). https://doi.org/10.1080/10426500902717333
F.A. Attaby, A.M. Abdel-Fattah, L.M. Shaif, M.M. Elsayed, Phosphorus Sulfur Silicon Relat. Elem. 185, 668–679 (2010). https://doi.org/10.1080/10426500902917644
Y. Feng, X. Wang, Oxid. Med. Cell Longev. 2012, 472932 (2012). https://doi.org/10.1155/2012/472932
M.E. McLellan, S.T. Kajdasz, B.T. Hyman, B.J. Bacskai, J. Neurosci. 23, 2212–2217 (2003). https://doi.org/10.1523/JNEUROSCI.23-06-02212.2003
S. Çakmak, S. Kansiz, M. Azam, C.C. Ersanlı, O. Idil, A. Veyisoğlu, H. Yakan, H. Kütük, A. Chutia, ACS Omega 7, 11320–11329 (2022). https://doi.org/10.1021/acsomega.2c00318
J.A. Arnott, S.L. Planey, Expert Opin. Drug Discov. 7, 863–875 (2012). https://doi.org/10.1517/17460441.2012.714363
C.A. Lipinski, Drug Discov. Today Technol. 1, 337–341 (2004). https://doi.org/10.1016/j.ddtec.2004.11.007
P.T. Thuong, T.M. Hung, T.M. Ngoc, D.T. Ha, B.S. Min, S.J. Kwack, T.S. Kang, J.S. Choi, K. Bae, Phytother. Res. 24, 101–106 (2010). https://doi.org/10.1002/ptr.2890
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ahmed, A.A.M., Mekky, A.E.M. & Sanad, S.M.H. New bis(pyrazolo[3,4-b]pyridines) and bis(thieno[2,3-b]pyridines) as potential acetylcholinesterase inhibitors: synthesis, in vitro and SwissADME prediction study. J IRAN CHEM SOC 19, 4457–4471 (2022). https://doi.org/10.1007/s13738-022-02614-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-022-02614-8