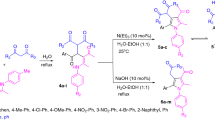
Abstract
A fast and convenient method for synthesis of some new functionalized pyrrole derivatives have been described via a three-component reaction between arylglyoxals, Meldrum’s acid and ethyl 2-chloro-3-(arylamino)but-2-enoate derivatives in excellent yields. When a mixture of arylglyoxal, Meldrum’s acid and ethyl 2-chloro-3-(arylamino)but-2-enoats were stirred in ethanol as a green solvent at room temperature, after one hour a solid product was separated from the reaction mixture. Simple filtration of this solid and its washing with ethanol afforded pure products. The structure of the products was proved by elemental analysis and IR and NMR apectral data.
Graphical abstract



Similar content being viewed by others
References
S.S. Gholap, Eur. J. Med. Chem 110, 13 (2016)
H. Fan, J. Peng, M.T. Hamann, J.F. Hu, Chem. Rev. 108, 264 (2008)
R.W. Bürli, D. McMinn, J.A. Kaizerman, W. Hu, Y. Ge, Q. Pack, V. Jiang, M. Gross, M. Garcia, R. Tanaka, H.E. Moser, Bioorg. Med. Chem. Lett. 14, 1253 (2004)
M.N. Narule, M.K. Gaidhane, P.K. Gaidhane, J. Pharm. Res. 6, 626 (2013)
C. Battilocchio, G. Poce, S. Alfonso, G.C. Porretta, S. Consalvi, L. Sautebin, S. Pace, A. Rossi, C. Ghelardini, L.D.C. Mannelli, S. Schenone, Bioorg. Med. Chem. 21, 3695 (2013)
S.D. Joshi, S.R. Dixit, M.N. Kirankumar, T.M. Aminabhavi, K.V.S.N. Raju, R. Narayan, C. Lherbet, K.S. Yang, Eur. J. Med. Chem. 107, 133 (2016)
A. Kamal, G. Ramakrishna, V.L. Nayak, P. Raju, A.S. Rao, A. Viswanath, M.V.P.S. Vishnuvardhan, S. Ramakrishna, G. Srinivas, Bioorg. Med. Chem. 20, 789 (2012)
M.W. Roomi, S.F. MacDonald, Can. J. Chem. 48, 1689 (1970)
F. Bonnaterre, M. Bois-Choussy, J. Zhu, Org. Lett. 8, 4351 (2006)
B. Wang, Y. Gu, C. Luo, T. Yang, L. Yang, J. Suo, Tetrahedron Lett. 45, 3369 (2004)
V. Estévez, M. Villacampa, J.C. Menéndez, Chem. Soc. Rev. 43, 4633 (2014)
D. Tzankova, S. Vladimirova, L. Peikova, M. Georgieva, J. Chem. Tech. Matallurgy 53, 451 (2018)
A. Sharma, P. Piplani, J. Heterocycl. Chem. 54, 27 (2017)
R.A. Jones, G.P. Bean, J. Heterocycl. Chem. 34, 27 (2013)
V. Bhardwaj, D. Gumber, V. Abbot, S. Dhiman, P. Sharma, RSC Adv. 5, 15233 (2015)
M. Bayat, S. Nasri, B. Notash, Tetrahedron 73, 1522 (2017)
X.B. Chen, Z.C. Liu, L.F. Yang, S.J. Yan, J. Lin, ACS Sustain Chem. Eng. 2, 1155 (2014)
D.J. Viradiya, B.H. Baria, R. Kakadiya, V.C. Kotadiya, Int. Lett. Chem Phy. Astro. 11, 257 (2014)
H. Wang, X. Liu, X. Feng, Z. Huang, D. Shi, GAP Green Chem. 15, 3307 (2013)
S. Ambethkar, V. Padmini, N. Bhuvanesh, New J. Chem. 40, 4705 (2016)
R. Mishra, A.K. Panday, L.H. Choudhury, J. Pal, R. Subramanian, A. Verma, Chem. Eur. J. 19, 2789 (2017)
J. Wei, L. Liu, D.N. Tang, C.P. Wu, X.J. Zhao, W.J. Hao, B. Jiang, J. Heterocycl. Chem. 54, 3403 (2017)
M. Ghandi, A. Jourablou, A. Abbasi, J. Heterocycl. Chem. 54, 3108 (2017)
S. Karamthulla, A. Jana, L.H. Choudhury, ACS Comb Sci. 19, 108 (2017)
N.N. Man, J.Q. Wang, L.M. Zhang, L.R. Wen, M. Li, J. Org. Chem. 82, 5566 (2017)
I. Dhinakaran, V. Padmini, N. Bhuvanesh, ACS Comb Sci. 18, 236 (2016)
M. Anary-Abbasinejad, M. Talebizadeh, J. Iran. Chem. Soc. 11, 963 (2014)
F. Mousavizadeh, M. Talebizadeh, M. Anary-Abbasinejad, Tetrahedron Lett. 59, 2970 (2018)
M. Masoudi, M. Anary-Abbasinejad, Tetrahedron Lett. 57, 103 (2016)
H.A. Riley, A.R. Gray, Organic Synthesis (Wiley, New York, 1943)
Funding
The funding was provided by vali-e-asr university of rafsanjan
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nezhad-shahrokhabadi, F., Anary-Abbasinejad, M. An efficient synthesis of polyfunctionalized pyrroles by three-component reaction of arylglyoxals, Meldrum’s acid and ethyl 2-chloro-3-(arylamino)but-2-enoates. J IRAN CHEM SOC 19, 2655–2661 (2022). https://doi.org/10.1007/s13738-021-02485-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-021-02485-5