Abstract
A novel series of 1,2,3-triazole-linked pyrazoline analogues were prepared by the reaction of 3-(4-(benzyloxy)phenyl)-1-(1-(arylphenyl)-5-methyl-1H-1,2,3-triazol-4-yl)prop-2-en-1-one with hydrazine hydrate in the presence of glacial acetic acid medium. The structures of the newly synthesized pyrazoline derivatives were established by elemental analysis, FT-IR, 1H NMR, 13C{1H} NMR, and mass spectral analysis. In addition, synthesized compounds DFT calculations and 3D structures of the synthesized compounds were performed using Gaussian 09 software, hybrid models and MM2 force techniques were used to obtain energy minimized structures. The antibacterial activities of the synthesized pyrazolines were determined against Gram-positive and Gram-negative strains. Among them, compounds with meta-chloro substitution on phenyl ring of pyrazoline showed the highest magnitude of inhibition against Pseudomonas aeruginosa and bromo substitution on para position of phenyl ring exhibited highest magnitude of inhibition against Staphylococcus aureus.
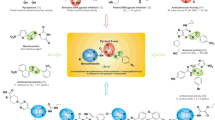
Graphic abstract







Similar content being viewed by others
References
R. Santosh, M.K. Selvam, S.U. Kanekar, G.K. Nagaraja, ChemistrySelect 3, 6338 (2018)
H. Harbottle, S. Thakur, S. Zhao, D.G. Anim, Biotechnol. 17, 111 (2006)
V. Kamat, S.P. Nayak, G. Adiga, C.H.A. Rajeena, S.U. Kanekar, P.D. Rekha, Heterocycl. Lett. 8, 671 (2018)
C.H.A. Rajeena, S.P. Nayak, G. Ganesh, J. Chem. Chem. Sci. 8, 49 (2018)
B.F. Abdel-Wahab, E. Abdel-Latif, H.A. Mohamed, G.E.A. Awad, Eur. J. Med. Chem. 52, 263 (2012)
R. Kant, V. Singh, G. Nath, S.K. Awasthi, A. Agarwal, Eur. J. Med. Chem. 124, 218 (2016)
M.H. Shaikh, D.D. Subhedar, L. Nawale, D. Sarkar, F.A.K. Khan, J.N. Sangshetti, B.B. Shingate, Med. Chem. Commun. 6, 1104 (2015)
K.T. Wong, H. Osman, T. Parumasivam, U. Supratman, M.T. Che Omar, M.N. Azmi, Molecules 26(7), 2081 (2021)
W.T. Li, W.H. Wu, C.H. Tang, R. Tai, S.T. Chen, A.C.S. Comb, Sci. 13, 72 (2011)
M. Patel, N. Pandey, J. Timaniya, P. Parikh, A. Chauhan, N. Jain, K. Patel, RSC Adv. 11(44), 27627 (2021)
B.S. Holla, M. Mahalinga, M.S. Karthikeyan, B. Poojary, P.M. Akberali, N.S. Kumari, Eur. J. Med. Chem. 40, 1173 (2005)
N.V. Sadgir, World J. Pharm. Res. 10(3), 2202 (2021)
S. Nagarajan, P. Shanmugavelan, M. Sathishkumar, R. Selvi, A. Ponnuswamy, H. Harikrishnan, V. Shanmugaiah, Chin. Chem. Lett. (2014). https://doi.org/10.1016/j.cclet.2013.12.017
M. Bhat, G.K. Nagaraja, P. Divyaraj, N. Harikrishna, S.R. Pai, S. Biswas, S.K. Peethamber, RSC Adv. (2016). https://doi.org/10.1039/c6ra22705h
N. Shankaraiah, S. Nekkanti, U.R. Brahma, N. PraveenKumar, N. Deshpande, D. Prasanna, K.R. Senwar, U.J. Lakshmi, Bioorg. Med. Chem. (2017). https://doi.org/10.1016/j.bmc.2017.07.031
C. Bagul, G.K. Rao, V.K.K. Makani, J.R. Tamboli, M. Pal-Bhadra, A. Kamal, Medchemcom (2013). https://doi.org/10.1039/C7MD00193B
S. Kumar, A. Saini, J. Gut, P.J. Rosenthal, R. Raj, V. Kumar, Eur. J. Med. Chem. (2017). https://doi.org/10.1016/j.ejmech.2017.07.041
B. Varghese, S.N. Al-Busafi, F.O. Suliman, M.Z. Salma, S.M. Al-Kindy, RSC Adv. 7, 46999 (2017)
K. Ajay Kumar, M. Govindaraju, Int. J. Chemtech. Res. 8, 313 (2015)
M. Alam, S.A.A. Nami, M. Parveen, D. Lee, S. Park, Chin. Chem. Lett. 23, 1039 (2012)
M.A. Ali, M.S. Yar, M. Kumar, G.S. Pandian, Nat. Prod. Res. 7, 575 (2007)
S.M. El-Moghazy, F.F. Barsoum, H.M. Abdel-Rahman, A.A. Marzouk, Med. Chem. Res. 21, 1722 (2012)
N.T. Pokhodylo, R.D. Savka, V.S. Matiichuk, N.D. Obushak, Russ. J. Gen. Chem. 2, 309 (2009)
C.H.A. Rajeena, S.P. Nayak, G. Ganesh, V. Kamat, B.C. Revanasiddappa, H. Kumar, Heterocycl. Lett. 8, 49 (2018)
Gussian 09, Revision D.01, M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H.P. Hratchian, A.F. Izmaylov, J. Bloino, G. Zheng, J.L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J.A. Montgomery, Jr., J.E. Peralta, F. Ogliaro, M. Bearpark, J.J. Heyd, E. Brothers, K.N. Kudin, V.N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J.C. Burant, S.S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J.M. Millam, M. Klene, J.E. Knox, J.B. Cross, V. Bakken, C.Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, R.L. Martin, K. Morokuma, V.G. Zakrzewski, G.A. Voth, P. Salvador, J.J. Dannenberg, S. Dapprich, A.D. Daniels, O. Farkas, J.B. Foresman, J.V. Ortiz, J. Cioslowski, D.J. Fox, Gaussian, Inc., Wallingford, CT (2013)
Acknowledgements
The authors are thankful to Director SAIF- Panjab University (Chandigarh) and to Head-DST PURSE and USIC (Mangalore University) for providing spectral data. Author Vinuta Kamat would like to thank DST for providing fellowship.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rajeena, C.H.A., Kamat, V., Patil, V.B. et al. Synthesis and antibacterial evaluation of pyrazolines carrying (benzyloxy)benzaldehyde moiety. J IRAN CHEM SOC 19, 1641–1650 (2022). https://doi.org/10.1007/s13738-021-02403-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-021-02403-9