Abstract
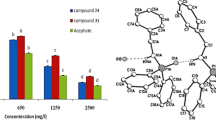
A series of phosphonic acid and bisphosphoramidate derivatives were synthesized and characterized. The bioactivities against the fungal pathogen Macrophomina phaseolina and human acetylcholinesterase AChE enzyme were studied using QSAR based on multiple linear regression. L17, with (p-Cl–C6H4–NH) (p-Cl–C6H4)C(H)P(O)(OC2H5)2 skeleton, demonstrated a great mortality on the M. phaseolina mycelial growth by 83% inhibition at 150 mg/L; the other tested derivative showed moderate to weak antifungal activity against the fungus. QSAR model based on the GA-MLR method revealed the importance of 3D descriptors (De, Mor18e, H8m, and Mor30p) on the antifungal activity. It showed good capability in predicting the fungicidal activity of the studied molecules. Another derivative, L5, with (m-CH3–NC5H4–NH)(m-CH3–C6H4)C(H)P(O)(OCH3)2 skeleton displays the most potent anti-AChE activity. The electronic parameters, ΔEL-H, and ELUMO, have the highest contribution of human AChE. The authors suggest that these models could be usefully employed in designing more effective crop protection compounds without side effects on non-target organisms.







Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in article main text and supplementary material.
References
R.P. Oliver, H.G. Hewitt, Fungicides in Crop Protection (Cabi, Wallingford, 2014).
J. Altman, Pesticide Interactions in Crop Production: Beneficial and Deleterious Effects (CRC Press, Boca Raton, 2018).
S. Kagale, T. Marimuthu, B. Thayumanavan, R. Nandakumar, R. Samiyappan, Antimicrobial activity and induction of systemic resistance in rice by leaf extract of Datura metel against Rhizoctonia solani and Xanthomonas oryzae pv. oryzae. Physiol. Mol. Plant Pathol. 65(2), 91–100 (2004)
G.M. Nicholson, Fighting the global pest problem: preface to the special toxicon issue on insecticidal toxins and their potential for insect pest control. Toxicon 49(4), 413–422 (2007)
C. Fest, K.J. Schmidt, The Chemistry of Organophosphorus Pesticides (Springer, Berlin, 2012).
A.M. Janssen, J.J.C. Scheffer, A.B. Svendsen, Antimicrobial activity of essential oils: a 1976–1986 literature review. Aspects of the test methods. Planta Med. 53(05), 395–398 (1987)
A. Grube, D. Donaldson, T. Kiely, L. Wu, Pesticides Industry Sales and Usage (US EPA, Washington, DC, 2011).
C.D. Tomlin, The Pesticide Manual: A World Compendium (Ed. 15) (British Crop Production Council, Hampsire, 2009).
G.F. Yang, X. Huang, Development of quantitative structure-activity relationships and its application in rational drug design. Curr. Pharm. Des. 12(35), 4601–4611 (2006)
S. Du, H. Lu, D. Yang, H. Li, X. Gu, C. Wan, C. Jia, M. Wang, X. Li, Z. Qin, Synthesis, antifungal activity and QSAR of some novel carboxylic acid amides. Molecules 20(3), 4071–4087 (2015)
Z.J. Zhang, Y. Zeng, Z.Y. Jiang, B.S. Shu, V. Sethuraman, G.H. Zhong, Design, synthesis, fungicidal property and QSAR studies of novel β-carbolines containing urea, benzoylthiourea and benzoylurea for the control of rice sheath blight. Pest Manag. Sci. 74(7), 1736–1746 (2018)
C. Hansch, T. Fujita, Classical and Three-Dimensional QSAR in Agrochemistry (American Chemical Society, Washington, DC, 1995), pp. 1–12
M. Karelson, V.S. Lobanov, A.R. Katritzky, Quantum-chemical descriptors in QSAR/QSPR studies. Chem. Rev. 96(3), 1027–1044 (1996)
J. Devillers, A.T. Balaban, Topological Indices and Related Descriptors in QSAR and QSPAR (CRC Press, Boca Raton, 2000).
R. Karbakhsh, R. Sabet, Application of different chemometric tools in QSAR study of azolo-adamantanes against influenza A virus. Res. Pharm. Sci. 6(1), 23 (2011)
M. de Candia, L. Summo, A. Carrieri, C. Altomare, A. Nardecchia, S. Cellamare, A. Carotti, Investigation of platelet aggregation inhibitory activity by phenyl amides and esters of piperidinecarboxylic acids. Bioorg. Med. Chem. 11(7), 1439–1450 (2003)
HyperChem, Hypercube, Inc., http://www.hyper.com
R. Todeschini, V. Consonni, A. Mauri, M. Pavan, Software Dragon: Calculation of Molecular Descriptors (Department of Environmental Sciences, University of Milano-Bicocca, Milan, 2003).
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H.P. Hratchian, A.F. Izmaylov, J. Bloino, G. Zheng, J.L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J.A. Montgomery Jr., J.E. Peralta, F. Ogliaro, M. Bearpark, J.J. Heyd, E. Brothers, K.N. Kudin, V.N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J.C. Burant, S.S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J.M. Millam, M. Klene, J.E. Knox, J.B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, R.L. Martin, K. Morokuma, V.G. Zakrzewski, G.A. Voth, Dannenberg JJ. Salvador, S. Dapprich, A.D. Daniels, O. Farkas, J.B. Foresman, J.V. Ortiz, J. Cioslowski, D.J. Fox, Gaussian 09, Revision D.01 (Gaussian Inc., Wallingford, CT, 2013).
SPSS for Windows, Version 10.05 (SPSS Inc., Chicago, 1999).
MATLAB, Version 7.10.0 (R2010a) (The MathWorks Inc, Natick, Massachusetts, 2010).
Bruker (2005) Programs APEX II, Version 2.0e1; SAINT, Version 7.23A; SADABS, Version 2004/1; XPREP, Version 2005/2; SHELXTL, Version 6.1, Bruker AXS Inc., Madison, WI, USA.
Agilent Technologies CrysAlis PRO, Yarnton, Oxfordshire, UK, 2010.
G.M. Sheldrick, Acta Crystallogr. A 64, 112–122 (2008)
K. Moedritzer, R.R. Irani, The Direct synthesis of α-aminomethylphosphonic acids. Mannich-type reactions with orthophosphorous acid. J Org. Chem. 31, 1603–1607 (1966)
G.L. Ellman, K.D. Courtney, V. Andres Jr., R.M. Featherstone, A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7(2), 88–95 (1961)
V. Mahdizadeh, N. Safaie, E.M. Goltapeh, Diversity of Macrophomina phaseolina based on morphological and genotypic characteristics in Iran. Plant Pathol. J. 27(2), 128–137 (2011)
H. Schmitz, Poisoned food technique. Ind. Eng. Chem. Analyst Ed. 2, 361 (1930)
L.S. Aiken, S.G. West, R.R. Reno, Multiple Regression: Testing and Interpreting Interactions (Sage, Beverley Hills, 1991).
K.S. Tang, K.F. Man, S. Kwong, Q. He, Genetic algorithms and their applications. IEEE Signal Process. Mag. 13(6), 22–37 (1996)
J. Bernstein, R.E. Davis, L. Shimoni, N.L. Chang, Patterns in hydrogen bonding: functionality and graph set analysis in crystals. Angew. Chem., Int. Ed. Engl. 34(15), 1555–1573 (1995)
R. Todeschini, V. Consonni, Handbook of Molecular Descriptors, vol 11 (Wiley, New York, 2008).
R. Leardi, Application of genetic algorithm–PLS for feature selection in spectral data sets. J. Chemom. 14(5–6), 643–655 (2000)
M. Stone, Cross-validatory choice and assessment of statistical predictions. J. Roy. Stat. Soc.: Ser. B (Methodol.) 36(2), 111–133 (1974)
J. Galvez, R. Garcia, M.T. Salabert, R. Soler, Charge indexes. New topological descriptors. J. Chem. Inf. Comput. Sci. 34(3), 520–525 (1994)
J. Devillers, A.T. Balaban (eds.), Topological Indices and Related Descriptors in QSAR and QSPAR (CRC Press, Boca Raton, 2000)
R. Todeschini, P. Gramatica, 3D-modelling and prediction by WHIM descriptors. Part 5. Theory development and chemical meaning of WHIM descriptors. Quant. Struct. Act. Relat. 16, 113–119 (1997)
R. Todeschini, M. Lasagni, E. Marengo, New molecular descriptors for 2D- and 3D-structures. Theory J. Chemom. 8, 263–273 (1994)
J. Gasteiger, J. Sadowski, J. Schuur, P. Selzer, L. Steinhauer, V. Steinhauer, Chemical information in 3D space. J. Chem. Inf. Comput. Sci. 36, 1030–1037 (1996)
J.H. Schuur, P. Selzer, J. Gasteiger, The coding of the three-dimensional structure of molecules by molecular transforms and its application to structure-spectra correlations and studies of biological activity. J. Chem. Inf. Comput. Sci. 36, 334–344 (1996)
H. Kušić, B. Rasulev, D. Leszczynska, J. Leszczynski, N. Koprivanac, Prediction of rate constants for radical degradation of aromatic pollutants in water matrix: a QSAR study. Chemosphere 75, 1128–1134 (2009)
M. Di Tullio, C. Maccallini, A. Ammazzalorso, L. Giampietro, R. Amoroso, B. De Filippis, M. Fantacuzzi, P. Wiczling, R. Kaliszan, QSAR, QSPR and QSRR in terms of 3-D-MoRSE descriptors for in silico screening of clofibric acid analogues. Mol. Inf. 31(6–7), 453–458 (2012)
L. Saíz-Urra, Y. Pérez-Castillo, M. Pérez González, R. Molina Ruiz, M.N.D. Cordeiro, J.E. Rodríguez-Borges, X. García-Mera, Theoretical prediction of antiproliferative activity against murine leukemia tumor cell line (L1210). 3D-morse descriptor and its application in computational chemistry. QSAR Combin. Sci. 28(1), 98–110 (2009)
V. Consonni, R. Todeschini, Rational Approaches to Drug Design (Prous Science, Barcelona, 2001), p. 235
V. Consonni, R. Todeschini, M. Pavan, Structure/response correlations and similarity/diversity analysis by GETAWAY descriptors. 1. Theory of the novel 3D molecular descriptors. J. Chem. Inf. Comput. Sci. 42(3), 682–692 (2002)
V. Consonni, R. Todeschini, M. Pavan, P. Gramatica, Structure/response correlations and similarity/diversity analysis by GETAWAY descriptors. 2. Application of the novel 3D molecular descriptors to QSAR/QSPR studies. J. Chem. Inf. Comput. Sci. 42(3), 693–705 (2002)
E. Estrada, E. Molina, 3D connectivity indices in QSPR/QSAR studies. J. Chem. Inf. Comput. Sci. 41(3), 791–797 (2001)
K. Gholivand, A.A.E. Valmoozi, M. Bonsaii, Synthesis, biological evaluation, QSAR study and molecular docking of novel N-(4-amino carbonylpiperazinyl)(thio) phosphoramide derivatives as cholinesterase inhibitors. Pestic. Biochem. Physiol. 112, 40–50 (2014)
M.B. Gholivand, L. Mohammadi-Behzad, G. Paimard, K. Gholivand, A.A.E. Valmoozi, Electrochemical characterization of some bisphosphoramidates spiked carbon paste electrodes and their applications in DNA sensing. J. Electroanal. Chem. 742, 62–69 (2015)
K. Gholivand, A.A. Ebrahimi Valmoozi, M. Rahimzadeh Dashtaki, F. Mohamadpanah, M. Dusek, V. Eigner, M. Pooyan, M. Bonsaii, M. Sharifi, M. Ghadamyari, Synthesis, crystal structure, fluorescence assay, molecular docking and QSAR/QSPR studies of Temephos derivatives as human and insect cholinesterase inhibitors. ChemistrySelect 2(28), 8828–8840 (2017)
K. Gholivand, L. Asadi, A.A.E. Valmoozi, M. Hodaii, M. Sharifi, H.M. Kashani, H.R. Mahzouni, M. Ghadamyari, A.A. Kalate, E. Davari, S. Salehi, Phosphorhydrazide inhibitors: toxicological profile and antimicrobial evaluation assay, molecular modeling and QSAR study. RSC Adv. 6(29), 24175–24189 (2016)
Acknowledgements
Financial support of this work by Iran National Science Foundation: INSF and Tarbiat Modares University is gratefully acknowledged.
Funding
This work was supported by Tarbiat Modares University.
Author information
Authors and Affiliations
Contributions
Gholivand, Valmoozi, Hosseini, Barzegar, Nasrollah Tabar, and Yaghoubi participated in the synthesis of the compounds and crystal structure analysis, Abbod provided the antifungal assay and the GA-MLR QSAR study of the fungicidal activity and wrote the related sections. Valmoozi were the major contributors in writing the manuscript, Safaie participated in the antifungal assay, Valmoozi provided the anti-AChE assay and related QSAR study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gholivand, K., Abbod, M., Ebrahimi Valmoozi, A.A. et al. Synthesis and crystal structure of phosphonic acid and bisphosphoramidate derivatives: QSAR studies of their anti-fungal potential on Macrophomina Phaseolina (Tassi) Goid. J IRAN CHEM SOC 18, 1591–1606 (2021). https://doi.org/10.1007/s13738-020-02133-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-020-02133-4