Abstract
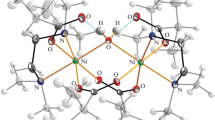
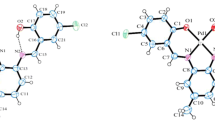
The crystal structure of 1-(4-methylphenyl)-3-(2-(trifluoromethyl)phenyl)triaz-1-ene 1-oxide (L) is monoclinic and has a space group of P21/c with a = 8.066(2) Å, b = 16.740(5) Å, c = 11.730(4) Å, β = 117.76(3)° and Z = 4. In this study, direct procedures were used to solve the crystalline structure of this complex and refine it by full-matrix least-squares to ultimate values of R1 = 0.0610 and wR2 = 0.1661 with 1474 reflections (I > 2σ(I)). The molecule is included in inter-hydrogen bonding with C5–H5 acting as donors and O atoms of N-oxide groups as acceptors (O1······H5) with a distance of 2.638 Å. These results were also confirmed by theoretical studies. The spectrophotometric titrations of the synthesized L with metal ions showed a substantially greater stability constant for its nickel ion complex with a mole ratio equal to 1. Consequently, the ligand was used for the selective extraction and spectrophotometric determining the Ni2+ ion in natural water. Under optimized conditions, the calibrating curve was linear over a nickel concentration range of 9.2 × 10−7–8.4 × 10−3 M. The detecting limit of this method was 6.0 × 10−7 M Ni2+. No considerable interference was found from at least 100 times concentrations of a number of possibly interfering ions.













Similar content being viewed by others
References
D.B. Kimball, R. Herges, M.M. Haley, Two unusual, competitive mechanisms for (2-ethynylphenyl) triazene cyclization: pseudocoarctate versus pericyclic reactivity. J. Am. Chem. Soc. 124, 1572–1573 (2002)
R. Meldola, F.W. Streatfeild, XLVI.—Researches on the constitution of azo-and diazo-derivatives. II. Diazoamido-compounds (continued). J. Chem. Soc. Trans. 51, 434–451 (1887)
N. Chen, M. Barra, I. Lee, N. Chahal, Substituent effects on the thermal cis-to-trans isomerization of 1, 3-diphenyltriazenes in aqueous solution. J. Org. Chem. 67(7), 2271–2277 (2002)
R. Kuroda, D.E. Wilman, 3-(4-Carbamoylphenyl)-1-methyltriazene 1-oxide. Acta Acta Cryst. C 41(10), 1543–1545 (1985)
B. Rezaei, L. Niromand, K. Alizadeh, P. Retailleau, Synthesis and crystal structure of 1-p-Tolyl-3-(3-(trifluoromethyl)phenyl)-triaz-1-ene 1-oxide. X-Ray Struct. Anal. Online 28, 37–38 (2012)
B. Rezaei, M. Fazlollahi, Synthesis, solvatochromism and crystal structure of 5-Methoxy-5, 6-diphenyl-4, 5-dihydro-2 H − 1, 2, 4-triazine-3-thione. Chem. Cent. J. 7(1), 130–134 (2013)
K. Alizadeh, B. Rezaei, E. Khazaeli, A new triazene-1-oxide derivative, immobilized on the triacetyl cellulose membrane as an optical Ni2 + sensor. Sens. Actuators, B 193, 267–272 (2014)
A.D. Becke, Phys. Rev. A 38, 3098 (1988)
C. Lee, W. Yang, R.G. Parr, Phys. Rev. B 37, 785 (1988)
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, G.A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. Marenich, J. Bloino, B.G. Janesko, R. Gomperts, B. Mennucci, H.P. Hratchian, J.V. Ortiz, A.F. Izmaylov, J.L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V.G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J.A. Montgomery Jr., J.E. Peralta, F. Ogliaro, M. Bearpark, J.J. Heyd, E. Brothers, K.N. Kudin, V.N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J.C. Burant, S.S. Iyengar, J. Tomasi, M. Cossi, J.M. Millam, M. Klene, C. Adamo, R. Cammi, J.W. Ochterski, R.L. Martin, K. Morokuma, O. Farkas, J.B. Foresman, D.J. Fox, GAUSSIAN 09 (Gaussian Inc, Wallingford CT, 2009)
G.M. Sheldrick, Program for the refinement of crystal structures. SHELXL97 (University of Gottingen, Germany, 1997)
J.L. Dye, V.A. Nicely, A general purpose curve fitting program for class and research use. J. Chem. Educ. 48(7), 443–447 (1971)
A.R. Ghiasvand, B. Rezaei, G.A. Masroor, Synthesis of a new hydroxytriazene derivative and its application for selective extraction-spectrophotometric determination of Nickel (II). Asian J. Chem. 18(3), 2185–2193 (2006)
Acknowledgement
It is my pleasure to express thanks to the Research Council of Lorestan University for their support with the completion of this research study. I wish to thank Prof. Datong Song from the University of Toronto, Chemistry Department, for his assistance and support. I would also like to thank Pascal Retailleau, Service de Cristallochimie, Cedex, France, for allowing the use of their X-ray facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Rezaei, B., Fazlollahi, M. Synthesis, crystal structure, theoretical study and application of 1-(4-methylphenyl)-3-(2- (trifluoromethyl)phenyl)triaz-1-ene 1-oxide in the extraction of Ni ions. J IRAN CHEM SOC 18, 1581–1590 (2021). https://doi.org/10.1007/s13738-020-02132-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-020-02132-5