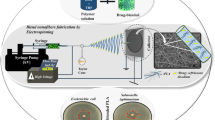
Abstract
Hydatid cyst is a common disease between humans and canines. Mebendazole has been used to treat this disease. Understanding and upgrading Mebendazole drug delivery system are the aim of this study. In this study, nanofibers containing drugs were fabricated by electrospinning. In the structure of nanofibers, polyvinylpyrrolidone (PVP) polymer is used as a drug release controller. In vitro, the living protoscoleces were exposed to nanofibers and the controlling effect of PVP was monitored using live protoscoleces mortality observation. The study found that PVP could be an effective controller of Mebendazole release from nanofiber. The higher the amount of PVP in the nanofibers, the lower the drug release rate and consequently the protoscoleces mortality rate decreases. Based on the statistical results of mortality, protoscoleces showed that PVP had significant controlling effects (p < 0.05). Kinetics and thermodynamic studies of the Mebendazole drug release process from nanofiber were performed. It was found that the release is carried out by the diffusion mechanism and the Sahlin–Peppas model describes the experimental data better than the other models. The thermodynamic parameters indicate that the enthalpy changes (ΔH > 0) are positive and that the process is endothermic. Entropy changes (ΔS < 0) indicate an increase in system disorder during drug release, and Gibbs free energy changes (ΔG > 0) indicate that drug release from the nanofibers is not spontaneous. Nanofibers that have more controller have a larger activation energy (Ea).






Similar content being viewed by others
References
S.B. Jomaa, N.H. Salem, I. Hmila, S. Saadi, A. Aissaoui, M. Belhadj, A. Chadly, Sudden death and hydatid cyst: a medicolegal study. Legal Med. 40, 17–21 (2019)
X. Chen, R. Zhang, T. Aji, Y. Shao, Y. Chen, H. Wen, Novel interventional management of hepatic hydatid cyst with nanosecond pulses on experimental mouse model. Sci. Rep. 7, 1–8 (2017)
S. Soltani, A. Rafiei, Z. Ramezani, M.R. Abbaspour, A. Jelowdar, M. Sagha Kahvaz, Evaluation of the hydatid cyst membrane permeability of albendazole and albendazole sulfoxide-loaded solid lipid nanoparticles. Jundishapur J. Nat. Pharm. Prod. 12, e34723 (2017)
B. Abedi, A.H. Maghsood, B. Khansarinejad, M. Fallah, M. Matini, S. Gholami, A.S. Pagheh, R. Ghasemikhah, Genotyping of Echinococcus granulosus isolates from livestock based on mitochondrial cox1 gene. J. Parasit. Dis. 43, 592–596 (2019)
A.S. Alharbi, Concurrent pulmonary and hepatic hydatid cysts managed with single stage surgery. Radiol. Case Rep. 14, 1306–1310 (2019)
E. Sozuer, M. Akyuz, S. Akbulut, Open surgery for hepatic hydatid disease. Int. Surg. 99, 764–769 (2014)
M.B. Rokni, Echinococcosis/hydatidosis in Iran. Iran. J. Parasitol. 4, 1–16 (2009)
P. Moro, P. Schantz, Echinococcosis: historical landmarks and progress in research and control. Ann. Trop. Med. Parasitol. 100, 703–714 (2006)
S. Ahmadnia, M. Moazeni, S. Mohammadi-Samani, A. Oryan, In vivo evaluation of the efficacy of albendazole sulfoxide and albendazole sulfoxide loaded solid lipid nanoparticles against hydatid cyst. Exp. Parasitol. 135, 314–319 (2013)
C. Cretu, R. Codreanu, B. Mastalier, L. Popa, I. Cordos, M. Beuran, D. SteriuIanulle, S. Simion, Albendazole associated to surgery or minimally invasive procedures for hydatid disease—how much and how long. Chirurgia (Bucur) 107, 15–21 (2012)
S.A. Pawluk, C.A. Roels, K.J. Wilby, M.H. Ensom, A review of pharmacokinetic drug–drug interactions with the anthelmintic medications albendazole and mebendazole. Clin. Pharmacokinet. 54, 371–383 (2015)
R.Y. Bai, V. Staedtke, C.M. Aprhys, G.L. Gallia, G.J. Riggins, Antiparasitic mebendazole shows survival benefit in 2 preclinical models of glioblastoma multiforme. Neuro-oncology 13, 974–982 (2011)
M. Moazeni, A. Nazer, In vitro effectiveness of garlic (Allium sativum) extract on scolices of hydatid cyst. World J. Surg. 34, 2677–2681 (2010)
S. Fathi, R. Ghasemikhah, R. Mohammadi, F. Tohidi, M. Sharbatkhori, Seroprevalence of hydatidosis in people referring to Reference laboratory of Gorgan, Golestan Province. Iran. J. Parasitol. 14, 436–443 (2019)
H. Mahmoudvand, M.F. Harandi, M. Shakibaie, M.R. Aflatoonian, N. ZiaAli, M.S. Makki, S. Jahanbakhsh, Scolicidal effects of biogenic selenium nanoparticles against protoscolices of hydatid cysts. Int. J. Surg. 12, 399–403 (2014)
B. Duan, X. Yuan, Y. Zhu, Y. Zhang, X. Li, Y. Zhang, K. Yao, A nanofibrous composite membrane of PLGA–chitosan/PVA prepared by electrospinning. Eur. Polym. J. 42, 2013–2022 (2006)
Y. Fu, L. Liu, R. Cheng, W. Cui, ECM decorated electrospun nanofiber for improving bone tissue regeneration. Polymers 10, 272 (2018)
P. Sasmal, P. Datta, Tranexamic acid-loaded chitosan electrospun nanofibers as drug delivery system for hemorrhage control applications. J. Drug Deliv. Sci. Technol. 52, 559–567 (2019)
Z. Wei, Z. Liu, X. Wang, S. Long, J. Yang, Smart carrier from electrospun core–shell thermo-sensitive ultrafine fibers for controlled drug release. Eur. Polym. J. 114, 1–10 (2019)
E. Sapountzi, M. Braiek, J.-F. Chateaux, N. Jaffrezic-Renault, F. Lagarde, Recent advances in electrospun nanofiber interfaces for biosensing devices. Sensors 17, 1887 (2017)
S. Fahimirad, F. Ajalloueian, Naturally-derived electrospun wound dressings for target delivery of bio-active agents. Int. J. Pharm. 566, 307–328 (2019)
Q. Wang, S. Liu, L. Fu, Z. Cao, W. Ye, H. Li, P. Guo, X. Zhao, Electrospun γ-Fe2O3 nanofibers as bioelectrochemical sensors for simultaneous determination of small biomolecules. Anal. Chim. Acta 1026, 125–132 (2018)
A. Akhgari, Z. Shakib, S. Sanati, A review on electrospun nanofibers for oral drug delivery. Nanomed. J. 4, 197–207 (2017)
K. Sasikanth, S. Nama, S. Suresh, B. Brahmaiah, Nanofibers—a new trend in nano drug delivery systems. Pharma Innov. 2, 118 (2013)
M. Eslamian, M. Khorrami, N. Yi, S. Majd, M.R. Abidian, Electrospinning of highly aligned fibers for drug delivery applications. J. Mater. Chem. B 7, 224–232 (2019)
K. Khoshnevisan, H. Maleki, H. Samadian, S. Shahsavari, M.H. Sarrafzadeh, B. Larijani, F.A. Dorkoosh, V. Haghpanah, M.R. Khorramizadeh, Cellulose acetate electrospun nanofibers for drug delivery systems: applications and recent advances. Carbohydr. Polym. 198, 131–141 (2018)
J. Weiss, K. Kanjanapongkul, S. Wongsasulak, T. Yoovidhya, Electrospun fibers: fabrication, functionalities and potential food industry applications, in Nanotechnology in the Food, Beverage and Nutraceutical Industries (Woodhead Publishing, 2012), pp. 362–397
Y. Liu, A. Nguyen, A. Allen, J. Zoldan, Y. Huang, J.Y. Chen, Regenerated cellulose micro-nano fiber matrices for transdermal drug release. Mater. Sci. Eng. C 74, 485–492 (2017)
H.K. Shaikh, R.V. Kshirsagar, S.G. Patil, Mathematical models for drug release characterization: a review. World J. Pharmacy Pharm. Sci. 4, 324–338 (2015)
G. Singhvi, M. Singh, Review: In vitro drug release characterization models. Int. J. Pharm. Stud. Res. 2, 77–84 (2011)
B. Yalagala, S. Khandelwal, J. Deepika, S. Badhulika, Wirelessly destructible MgO–PVP–graphene composite based flexible transient memristor for security applications. Mater. Sci. Semicond. Process. 104, 104673–104683 (2019)
R. Mishra, R. Varshney, N. Das, D. Sircar, P. Roy, Synthesis and characterization of gelatin–PVP polymer composite scaffold for potential application in bone tissue engineering. Eur. Polym. J. 119, 155–168 (2019)
R. Poonguzhali, S.K. Basha, V.S. Kumari, Fabrication of asymmetric nanostarch reinforced Chitosan/PVP membrane and its evaluation as an antibacterial patch for in vivo wound healing application. Int. J. Biol. Macromol. 114, 204–213 (2018)
H. Tian, Y. Liang, D. Yang, Y. Sun, Characteristics of PVP–stabilised NZVI and application to dechlorination of soil–sorbed TCE with ionic surfactant. Chemosphere 239, 124807–124815 (2020)
P. Costa, J.M.S. Lobo, Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 13, 123–133 (2001)
Y. Zhang, M. Huo, J. Zhou, A. Zou, W. Li, C. Yao, S. Xie, DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 12, 263–271 (2010)
M.C.L.C. Freire, F. Alexandrino, H.R. Marcelino, P.H.D.S. Picciani, K.G.D.H.E. Silva, J. Genre, A.G.D. Oliveira, E.S.T.D. Egito, Understanding drug release data through thermodynamic analysis. Materials 10, 651–669 (2017)
S. Dash, P.N. Murthy, L. Nath, P. Chowdhury, Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 67, 217–223 (2010)
R.S. Bhattarai, R.D. Bachu, S.H.S. Boddu, S. Bhaduri, Biomedical applications of electrospun nanofibers: drug and nanoparticle delivery. Pharmaceutics 1, 1–30 (2018)
U.E. Illangakoon, D.-G. Yu, B.S. Ahmad, N.P. Chatterton, G.R. Williams, 5-Fluorouracil loaded Eudragit fibers prepared by electrospinning. Int. J. Pharm. 495, 895–902 (2015)
C. Bounioux, R. Avrahami, G. Vasilyev, N. Patil, E. Zussman, R. Yerushalmi-Rozen, Single-step electrospinning of multi walled carbon nanotubes–poly(3-octylthiophene) hybrid nano-fibers. Polymer 86, 15–21 (2016)
P. Kampalanonwat, P. Supaphol, G.E. Morlock, Electrospun nanofiber layers with incorporated photoluminescence indicator for chromatography and detection of ultraviolet-active compounds. J. Chromatogr. A 1299, 110–117 (2013)
M. Eskandari, Y. Yamini, L. Fotouhi, S. Seidi, Microextraction of mebendazole across supported liquid membrane forced by pH gradient and electrical field. J. Pharm. Biomed. Anal. 54, 1173–1179 (2011)
D. Carson, Y. Jiang, K.A. Woodrow, Tunable release of multiclass anti-HIV drugs that are water-soluble and loaded at high drug content in polyester blended electrospun fibers. Pharm. Res. 33, 125–136 (2016)
S. Ansari, M. Karimi, Recent progress, challenges and trends in trace determination of drug analysis using molecularly imprinted solid-phase microextraction technology. Talanta 164, 612–625 (2017)
M.M. Markoski, E.S. Trindade, G. Cabrera, A. Laschuk, N. Galanti, A. Zaha, H.B. Nader, H.B. Ferreira, Praziquantel and albendazole damaging action on in vitro developing Mesocestoides corti (Platyhelminthes: Cestoda). Parasitol. Int. 55, 51–61 (2006)
S. Chaudhary, T. Garg, G. Rath, R.R. Murthy, A.K. Goyal, Enhancing the bioavailability of mebendazole by integrating the principles solid dispersion and nanocrystal techniques, for safe and effective management of human echinococcosis. Artif. Cells Nanomed. Biotechnol. 44, 937–942 (2016)
H.B. Dugstad, N.J. Petersen, H. Jensen, C. Gabel-Jensen, S.H. Hansen, S. Pedersen-Bjergaard, Development and characterization of a small electromembrane extraction probe coupled with mass spectrometry for real-time and online monitoring of in vitro drug metabolism. Anal. Bioanal. Chem. 406, 421–429 (2014)
X. Qi, T. Su, X. Tong, W. Xiong, Q. Zeng, Y. Qian, Z. Zhou, X. Wu, Z. Li, L. Shen, X. He, Facile formation of salecan/agarose hydrogels with tunable structural properties for cell culture. Carbohydr. Polym. 224, 115208–115216 (2019)
X. Qi, Z. Li, L. Shen, T. Qin, Y. Qian, S. Zhao, M. Liu, Q. Zeng, J. Shen, Highly efficient dye decontamination via microbial salecan polysaccharide-based gels. Carbohydr. Polym. 219, 1–11 (2019)
X. Qi, L. Lin, L. Shen, Z. Li, T. Qin, Y. Qian, X. Wu, X. Wei, Q. Gong, J. Shen, Efficient decontamination of lead ions from wastewater by salecan polysaccharide-based hydrogels. ACS Sustain. Chem. Eng. 7, 11014–11023 (2019)
X. Qi, Y. Yuan, J. Zhang, J.W. Bulte, W. Dong, Oral administration of salecan-based hydrogels for controlled insulin delivery. J. Agric. Food Chem. 66, 10479–10489 (2018)
X. Qi, R. Liu, M. Chen, Z. Li, T. Qin, Y. Qian, S. Zhao, M. Liu, Q. Zeng, J. Shen, Removal of copper ions from water using polysaccharide-constructed hydrogels. Carbohydr. Polym. 209, 101–111 (2019)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tavakoli, F., Shafiei, H. & Ghasemikhah, R. The effect of PVP application on Mebendazole release from electrospun nanofibers, kinetic study and thermodynamic analysis. J IRAN CHEM SOC 18, 1093–1102 (2021). https://doi.org/10.1007/s13738-020-02090-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-020-02090-y