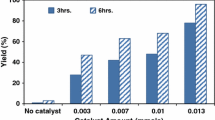
Abstract
Homogeneous liquid-phase oxidation of alkenes (allylbenzene, cis-cyclooctene, 4-chlorostyrene, styrene, 2-norbornene, 1-methyl cyclohexene, indene, lemonine, and 1-hexene) were catalyzed by using vanadium complex [VO(hyap)(acac)2] in existence of H2O2. The complex [VO(hyap)(acac)2] was formed as a crystal by the reaction of [VO(acac)2] and 2-hydroxyacetophenone (hyap) in the presence of methanol by refluxing the reaction mixture. Various analytical and spectroscopic techniques, namely FTIR, ESI–MS, UV–Vis, single-crystal XRD, and EPR, were used to analyze and optimize the structure of the complexes.
Graphic abstract






Similar content being viewed by others
References
G. Strukul, Catalytic Oxidations with Hydrogen Peroxide as Oxidant (Kluwer Academic, Dordrecht, 1992)
F.P. Ballistreri, C.M. Gangemi, A. Pappalardo, G.A. Tomaselli, R.M. Toscano, G.T. Sfrazzetto, Int. J. Mol. Sci. 17, 1112 (2016)
S.H. Kashani, M. Moghadam, S. Tangestaninejad, V. Mirkhani, I.M. Baltork, Catal. Lett. 148, 1110 (2018)
Y. Zhu, Q. Wang, R.G. Cornwall, Y. Shi, Chem. Rev. 114, 8199 (2014)
Y. Shen, P. Jiang, P.T. Wai, Q. Gu, W. Zhang, Catalysts 9, 31 (2019)
K.C. Gupta, A.K. Sutar, Coord. Chem. Rev. 252, 1420 (2008)
K.C. Gupta, A.K. Sutar, C.C. Lin, Coord. Chem. Rev. 253, 1936 (2009)
K.C. Gupta, A.K. Sutar, J. Mol. Catal. 272, 64 (2007)
A.G.J. Ligtenbarg, R. Hage, B.L. Feringa, Coord. Chem. Rev. 237, 89 (2003)
F.V. de Velde, I.W.C.E. Arends, R.A. Sheldon, J. Inorg. BioChem. 80, 81 (2000)
D.F. Back, G.M. de Oliveira, D. Roman, M.A. Ballin, R. Kober, P.C. Piquini, Inorg. Chim. Acta 412, 6 (2014)
H.H. Monfared, R. Bikas, P. Mayer, Inorg. Chim. Acta 363, 2574 (2010)
M.R. Maurya, Coord. Chem. Rev. 383, 43 (2019)
M. Sutradhar, L.M.D.R.S. Martins, M.F.C.G. da Silva, A.J.L. Pombeiro, Coord. Chem. Rev. 301, 200 (2015)
M.R. Maurya, C. Haldar, A. Kumar, M.L. Kuznetsov, F. Avecillac, J.C. Pessoa, Dalton Trans. 42, 11941 (2013)
T. Hirao, Coord. Chem. Rev. 237, 1 (2003)
T. Hirao, Chem. Rev. 97, 2707 (1997)
A.E. Shilov, G.B. Shul’pin, Chem. Rev. 97, 2879 (1997)
B.G. Świerkosz, F. Trifirò, Appl. Catal. A 157, 1 (1997)
A. Butler, M.J. Clague, G.E. Meister, Chem. Rev. 94, 625 (1994)
U. Schuchardt, M.C. Guerreiro, G.B. Shul’pin, Russ. Chem. Bull. 47, 247 (1998)
O. Bortolini, F. Di Furia, P. Scrimin, G. Modena, J. Mol. Catal. 7, 59 (1980)
E.J. Eisenbraun, A.R. Bader, J.W. Polacheck, E. Reif, J. Org. Chem. 28, 2057 (1963)
R. Neumann, M. Levin-Elad, Appl. Catal. A 122, 85 (1995)
D. Wei, W. Chuei, G.L. Haller, Catal. Today 51, 501 (1999)
G. Bellussi, M.S. Rigutto, Stud. Surf. Sci. Catal. 85, 177 (1994)
A.M. Khenkin, L. Weiner, Y. Wang, R. Neumann, J. Am. Chem. Soc. 123, 8531 (2001)
R.D. Gall, M. Faraj, C.L. Hill, Inorg. Chem. 33, 5015 (1994)
T. Bunchuay, R. Ketkaew, P. Chotmongkolsap, T. Chutimasakul, J. Kanarat, Y. Tantirungrotechai, J. Tantirungrotechai, Catal. Sci. Technol. 7, 6069 (2017)
MdM Hossain, S.G. Shyu, Tetrahedron 70, 251 (2014)
Z.P. Pai, N.V. Selivanova, P.V. Oleneva, P.V. Berdnikova, A.M. Beskopyl’nyi, Catal. Commun. 88, 45–49 (2017)
A. Maurya, N. Kesharwani, P. Kachhap, V.K. Mishra, N. Chaudhary, C. Haldar, Appl. Organomet. Chem. 33(9), e5094 (2019)
K. Parida, K.G. Mishra, S.K. Dash, Ind. Eng. Chem. Res. 51, 2235 (2012)
SAGE, Int. J. Toxicol. 25, 11 (2006)
P.J. Hajduk, M. Bures, J. Praestgaard, S.W. Fesik, J. Med. Chem. 43, 3443 (2000)
C. Ballatore, D.M. Huryn, A.B. Smith, ChemMedChem 8, 385 (2013)
S. Takizawa, T. Katayama, H. Somei, Y. Asano, T. Yoshida, C. Kameyama, D. Rajesh, K. Onitsuka, T. Suzuki, M. Mikami, H. Yamataka, D. Jayaprakash, H. Sasai, Tetrahedron 64, 3361 (2008)
M.R. Maurya, R. Tomar, L. Rana, F. Avecilla, Eur. J. Inorg. Chem. 2018, 2952 (2018)
M.R. Maurya, B. Sarkar, F. Avecilla, I. Correia, Dalton Trans. 45, 17343 (2016)
Á. Kupán, J. Kaizer, G. Speier, M. Giorgi, M. Réglier, F. Pollreisz, J. Inorg. Biochem. 103, 389 (2009)
M.R. Maurya, N. Chaudhary, F. Avecilla, P. Adão, J.C. Pessoa, Dalton Trans. 44, 1211 (2015)
M.R. Maurya, N. Chaudhary, F. Avecilla, Polyhedron 67, 436 (2014)
V.K. Singh, A. Maurya, N. Kesharwani, P. Kachhap, S. Kumari, A.K. Mahato, V.K. Mishra, C. Haldar, J. Coord. Chem. 71(4), 520 (2018)
P.M. Anarjan, R. Bikas, H.H. Monfared, P.A. Kevych, P. Mayer, J. Mol. Struct. 1131, 258 (2017)
S.S. Amin, K. Cryer, B. Zhang, S.K. Dutta, S.S. Eaton, O.P. Anderson, S.M. Miller, B.A. Reul, S.M. Brichard, D.C. Crans, Inorg. Chem. 39, 406 (2000)
S. Meijers, V. Ponec, E. Finocchio, G. Busca, J. Chem. Soc. Faraday Trans. 91, 1861 (1995)
J. Zhao, L. Liu, S. Xiang, Q. Liu, H. Chen, Org. Biomol. Chem. 13, 5613 (2015)
G. Romanowski, J. Mol. Catal. A Chem. 368, 137 (2013)
M.R. Maurya, M. Kumar, S. Sikarwar, React. Funct. Polym. 66, 808 (2006)
M.R. Maurya, A. Kumar, M. Ebel, D. Rehder, Inorg. Chem. 45, 15 (2006)
M.M. Javadi, M. Moghadam, I.M. Baltork, S. Tangestaninejad, V. Mirkhani, J. Iran. Chem. Soc. 12, 477 (2015)
M. Sedighipoor, A.H. Kianfar, W.A.K. Mahmood, M.H. Azarian, Inorg. Chim. Acta 457, 116 (2017)
M.C. Hsiao, S.T. Liu, Catal. Lett. 139, 61 (2010)
N. Mizuno, K. Kamata, Coord. Chem. Rev. 255, 2358 (2011)
N. Mizuno, Y. Nakagawa, K. Yamaguchi, J. Mol. Catal. A Chem. 251, 286 (2006)
G. Grivani, A.D. Khalaji, V. Tahmasebi, K. Gotoh, H. Ishida, Polyhedron 31, 265 (2012)
P. Paula, A. Ghosh, S. Chatterjee, A. Bera, S.M. Alamb, SkM Islam, Inorg. Chim. Acta 492, 198 (2019)
C. Weerakkody, S. Biswas, W. Song, J. He, N. Wasalathanthri, S. Dissanayake, D.A. Kriz, B. Dutta, S.L. Suib, Appl. Catal. B Environ. 22(1), 681 (2018)
A. Nodzewska, A. Wadolowska, M. Watkinson, Coord. Chem. Rev. 382, 181 (2019)
R.A. Rowe, M.M. Jones, Inorg. Synth. 5, 113 (1957)
G.M. Sheldrick, SHELXS-97, Program of Crystal Structure Solution (University of Göttingen, Germany, 1997)
C. Arunagiri, A.G. Anitha, A. Subashini, S. Selvakumar, N.K. Lokanath, Chem. Data Collect. 17, 169 (2018)
G.M. Sheldrick, SHELX2017, Programs for Crystal Structure Determination (Universität Göttingen, Germany, 2017)
Acknowledgements
A. M. thanks Dr. C. Haldar, department of chemistry, IIT (ISM), Dhanbad, for providing instrumental facility. The author acknowledges IIT (ISM), Dhanbad, Jharkhand, for fellowship. The author acknowledges the central research facility, IIT (ISM), Dhanbad, for providing single-crystal XRD analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maurya, A. Homogeneous catalytic oxidation of alkenes employing mononuclear vanadium complex with hydrogen peroxide. J IRAN CHEM SOC 17, 3261–3269 (2020). https://doi.org/10.1007/s13738-020-01988-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-020-01988-x