Abstract
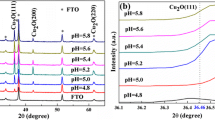
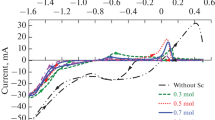
The electrochemical behavior of a copper oxide electrode produced by annealing and electrochemical methods was studied in an acetonitrile solvent by means of the cyclic voltammetry method. The presence of different peaks of oxidation and reduction produced by repeating the potential scans, numerous variations in the current, and shifts of peak potentials in consecutive cycles have been justified. Voltammograms proved that various oxidation species can be produced in solid-deposited forms of Cu2Os and CuOs and dissolved forms of Cu(II)sol and Cu(I)sol ions. The experimental results indicated that higher amounts of Cu2Os than CuOs can be produced in the process of copper electrode annealing. Also, the nature of copper species is responsible for different peak currents in the cyclic voltammograms, characterized by UV–Vis and XRD spectrometric methods.








Similar content being viewed by others
References
G. Papadimitropoulos, N. Vourdas, V.E. Vamvakas, D. Davazoglou, Thin Solid Films 515, 2428 (2006)
A.K. Mukhopadhyay, A. Chakraborty, A. Chatterjee, S.K. Lahiri, Thin Solid Films 209, 92 (1992)
X. Jiang, T. Herricks, Y. Xia, Nano Lett. 2, 1333 (2002)
J. Klunker, W. Schäfer, J. Electroanal. Chem. 466, 107 (1999)
S.E. Allen, R.R. Walvoord, R. Padilla-Salinas, M.C. Kozlowski, Chem. Rev. 113, 6234 (2013)
D.B. Rorabacher, R.R. Schroeder, Electrochemistry of Copper (Wiley-VCH Verlag GmbH & Co. KGaA, 2007)
A.E. Rakhshani, Solid-State Electron. 29, 7 (1986)
D. Ren, Y. Deng, A.D. Handoko, C.S. Chen, S. Malkhandi, B.S. Yeo, ACS Catal. 5, 2814 (2015)
K.H.V. Reddy, V.P. Reddy, J. Shankar, B. Madhav, B.A. Kumar, Y. Nageswar, Tetrahedron Lett. 52, 2679 (2011)
M. Gupta, J. Iran. Chem. Soc. 13, 231 (2016)
J.B. Reitz, E.I. Solomon, J. Am. Chem. Soc. 120, 11467 (1998)
V.P. Reddy, A.V. Kumar, K. Swapna, K.R. Rao, Org. Lett. 11, 951 (2009)
Y. Guo, M. Dai, Z. Zhu, Y. Chen, H. He, T. Qin, Appl. Surf. Sci. 480, 601 (2019)
Z. Wang, Y. Zhang, H. Xiong, C. Qin, W. Zhao, X. Liu, Sci. Rep. 8, 6530 (2018)
M. Li, Y. Li, Q. Zhang, C. Qin, W. Zhao, Z. Wang, A. Inoue, Appl. Surf. Sci. 483, 285 (2019)
X. Yue, X. Luo, Z. Zhou, Y. Wu, Y. Hong Bai, New J. Chem. 43, 4947 (2019)
Y. Fan, P.F. Liu, Z.J. Yang, Ionics 21, 185 (2015)
M. Mirzaee, C. Dehghanian, J. Iran. Chem. Soc. 16, 283 (2019)
S.Y. Kim, C.H. Ahn, J.H. Lee, Y.H. Kwon, S. Hwang, J.Y. Lee, H.K. Cho, A.C.S. Appl, Mater. Interfaces 5, 2417 (2013)
S. Brittman, Y. Yoo, N.P. Dasgupta, S.I. Kim, B. Kim, P. Yang, Nano Lett. 14, 4665 (2014)
H. Wei, H. Gong, L. Chen, M. Zi, B. Cao, J. Phys. Chem. C 116, 10510 (2012)
M. Ristov, G. Sinadinovski, M. Mitreski, Thin Solid Films 167, 309 (1988)
J.K. Barton, A.A. Vertegel, E.W. Bohannan, J.A. Switzer, Chem. Mater. 13, 952 (2001)
T.D. Golden, M.G. Shumsky, Y. Zhou, R.A. VanderWerf, R.A. Van Leeuwen, J.A. Switzer, Chem. Mater. 8, 2499 (1996)
M. Ristov, G. Sinadinovski, I. Grozdanov, Thin Solid Films 123, 63 (1985)
Y. Nicolau, Appl. Surf. Sci. 22, 1061 (1985)
J. Zhao, X. Shu, Y. Wang, C. Yu, J. Zhang, J. Cui, Y. Qin, H. Zheng, J. Liu, Y. Zhang, Coat. Technol. Res. 299, 15 (2016)
Y. Li, S. Chang, X. Liu, J. Huang, J. Yin, G. Wang, D. Cao, Electrochim. Acta 85, 393 (2012)
Y. Yu, Y. Shi, C.H. Chen, Nanotechnology 18, 055706 (2007)
A.K. Potbhare, R.G. Chaudhary, P.B. Chouke, S. Yerpude, A. Mondal, V.N. Sonkusare, A.R. Rai, H.D. Juneja, Mater. Sci. Eng. C 99, 783 (2019)
K. Shiny, R. Sundararaj, N. Mamatha, B. Lingappa, MaderasCienc Tecnol 21, 1 (2019)
S. Biallozor, Electrochim. Acta 17, 1243 (1972)
A.I. Danilov, J.E.T. Andersen, E. Molodkina, Y.M. Polukarov, P. Møller, J. Ulstrup, Electrochim. Acta 43, 733 (1998)
C. Ji, G. Oskam, P.C. Searson, J. Electrochem. Soc. 148, C746 (2001)
O. Chyan, T.N. Arunagiri, T. Ponnuswamy, J. Electrochem. Soc. 150, C347 (2003)
J.L. Rosa, A. Robin, M. Silva, C.A. Baldan, M.P. Peres, J. Mater. Process. Technol. 209, 1181 (2009)
M.C. Figueiredo, I. Ledezma-Yanez, M.T. Koper, ACS Catal. 6, 2382 (2016)
R.D. Braun, Anal. Chim. Acta 99, 325 (1978)
R.D. Braun, Anal. Chim. Acta 120, 111 (1980)
L. Sestili, C. Furlani, A. Ciana, F. Garbassi, Electrochim. Acta 15, 225 (1970)
I. Kolthoff, J. Coetzee, J. Am. Chem. Soc. 79, 1852 (1957)
C. Furlani, L. Sestili, A. Ciana, F. Garbassi, Electrochim. Acta 12, 1393 (1967)
R.C. Larson, R.T. Iwamoto, J. Am. Chem. Soc. 82, 3239 (1960)
S. Min, X. Yang, A.Y. Lu, C.C. Tseng, M.N. Hedhili, L.J. Li, K.W. Huang, Nano Energy 27, 121 (2016)
D. He, G. Wang, G. Liu, H. Suo, C. Zhao, Dalton Trans. 46, 3318 (2017)
S.K. Lee, H.C. Hsu, W.H. Tuan, Mater. Res. 19, 51 (2016)
C.W. Li, M.W. Kanan, J. Am. Chem. Soc. 134, 7231 (2012)
A. Moreira, A.V. Benedetti, P. Cabot, P. Sumodjo, Electrochim. Acta 38, 981 (1993)
D. Tromans, R.H. Sun, J. Electrochem. Soc. 138, 3235 (1991)
K. Izutsu, Electrochemistry in Nonaqueous Solutions (Wiley, New York, 2009)
N. Topnani, S. Kushwaha, T. Athar, Int. J. Green Nanotechnol. Mater. Sci. Eng. 1, 67 (2009)
Acknowledgements
The authors are grateful for the support of the Iran National Science Foundation, INSF, under Grant No. 96004700.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohammadzadeh, S., Zare, H.R. & Khoshro, H. Electrochemical study of Cu2O/CuO composite coating produced by annealing and electrochemical methods. J IRAN CHEM SOC 16, 2719–2729 (2019). https://doi.org/10.1007/s13738-019-01736-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01736-w