Abstract
In this abstract, we discuss the progress related to sulfonated carbon-based materials in various acid-catalyzed organic transformations which are then further utilized in medicinal field, laboratories and industries. A simple and novel methodology was employed to prepare carbon-based nanocatalyst, i.e. cellulose[2-(sulfooxy)ethyl]mercaptosulfonic acid as a solid acid catalyst. This nanocatalyst is recyclable and also exhibited very high activity. Novel carbon-based nanocatalyst, i.e. cellulose[2-(sulfooxy)ethyl]mercaptosulfonic acid[SEMSA] was successfully synthesized by reacting mercaptoethanol and chlorosulfonic acid, and the catalytic activity of the prepared catalysts was evaluated for the one-pot synthesis of gem-bisamides from various aldehydes and benzamide, hexahydroacridine-1,8-diones via three-component condensation of aromatic aldehyde, dimedone and ammonium acetate or aromatic amine and 1,8-dioxo-octahydroxanthenes using aldehyde and dimedone. Application of this new heterogeneous nanocatalyst system offered the advantages of high yields, short reaction times, eco-friendly nature and easy work-up procedure compared to the conventional methods of the synthesis, and confirmation of products synthesized has been done using studies like 1H NMR and 13C NMR. Among the various catalysts, this nanocatalyst, i.e. cellulose [2-(sulfooxy)ethyl]mercaptosulfonic acid[SEMSA], was found to be the most active and selective and could be recycled several times without significant loss of activity. Also, scanning electron microscopy and transmission electron microscopy of the catalyst have been performed to know the internal and external morphology, size, thermo-gravimetric analysis to study the thermal stability and Fourier transform infrared spectroscopy to study the modification pattern of the catalyst have been undertaken and presented in this work. Due to its simple and inexpensive solid support, i.e. cellulose and environmentally benign toluene, ethanol and acetonitrile as solvents in three different transformations which are less toxic, easily available and less expensive than other solvents.
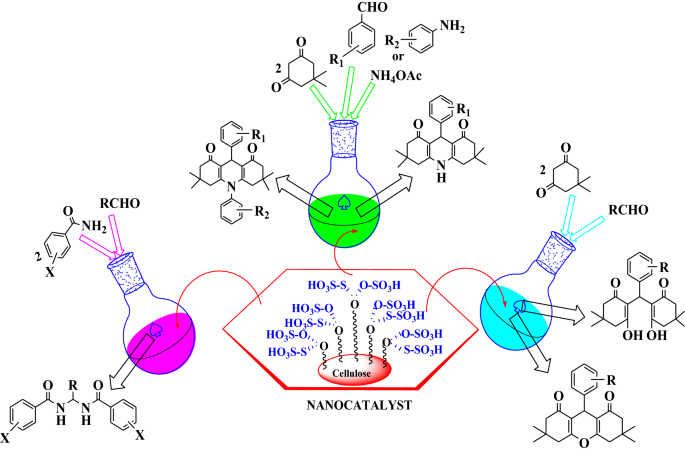
Graphic abstract
We reported here a novel route for the synthesis of gem-bisamides, hexahydroacridine-1,8-diones and 1,8-dioxo-octahydroxanthenes














Similar content being viewed by others
References
G. Kour, M. Gupta, Dalton Trans. 46, 7039 (2017)
J.H. Clark, Pure Appl. Chem. 73, 103–111 (2001)
J.A. Melero, R. Grieken, G. Morales, Chem. Rev. 106, 3790–3812 (2006)
S. Pathak, K. Debnath, A. Pramanik, Beilstein J. Org. Chem. 9, 2344–2353 (2013)
M. Kour, S. Paul, New J. Chem. 39, 6338–6350 (2015)
J. Cybulski, M. Cybulski, M. Sharma, R.A. Sheldon, Fine Chemicals Manufacture: Technology and Engineering, 1st edn. (Elsevier, Amsterdam, 2001)
D.M. Pore, U.V. Desai, T.S. Thopate, P.P. Wadgaonka, Synth. Commun. 34, 2135–2142 (2004)
A.D. Sagar, S.N. Chamle, M.V. Yadav, J. Chem. Pharm. Res. 5, 156–160 (2013)
J.H. Clark, Acc. Chem. Res. 35, 791–797 (2002)
M.R. Mohammadizadeh, A. Hasaninejad, M. Bahramzadeh, Z.S. Khanjarlu, Synth. Commun. 39, 1152–1165 (2009)
S.N. Sadat, F. Hatamjafari, Orient. J. Chem. 31, 1191–1193 (2015)
A. Maleki, M. Kamalzare, Tetrahedron Lett. 55, 6931–6934 (2014)
A. Maleki, H. Movahed, P. Ravaghi, T. Kari, RSC Adv. 6, 98777–98787 (2016)
A. Maleki, M. Aghaei, R. Paydar, J. Iran. Chem. Soc. 14, 485–490 (2017)
A. Maleki, P. Ravaghi, M. Aghaei, H. Movahed, Res. Chem. Intermed. 43, 5485–5494 (2017)
A. Maleki, H. Movahed, P. Ravaghi, Carbohydr. Polym. 156, 259–267 (2017)
A. Maleki, A.A. Jafari, S. Yousefi, Carbohydr. Polym. 175, 409–416 (2017)
A. Maleki, P. Ravaghi, H. Movahed, Micro Nano Lett. 13, 591–594 (2018)
A. Maleki, Ultrason. Sonochem. 40, 460–464 (2017)
A. Maleki, V. Eskandarpour, J. Rahimi, N. Hamidi, Carbohydr. Polym. 208, 251–260 (2018)
W.J. Lee, U.N. Maiti, J.M. Lee, J. Lim, T.H. Han, S.O. Kim, Chem. Commun. 50, 6818–6830 (2014)
M. Okamura, A. Takagaki, M. Toda, J.N. Kondo, K. Domen, T. Tatsumi, M. Hara, S. Hayashi, Chem. Mater. 18, 3039–3045 (2006)
M. Toda, A. Takagaki, M. Okamura, J.N. Kondo, S. Hayashi, K. Hayashi, M. Hara, Nature 438, 178 (2005)
A. Takagaki, M. Takagaki, M. Okamura, J.N. Kondo, S. Hayashi, K. Domen, M. Hara, Catal. Today 116, 157–161 (2006)
S. Suganuma, K. Nakajima, M. Kitano, D. Yamaguchi, H. Kato, S. Hayashi, M. Hara, J. Am. Chem. Soc. 130, 12787–12793 (2008)
S. Suganuma, K. Nakajima, M. Kitano, D. Yamaguchi, H. Kato, S. Hayashi, M. Hara, Solid State Sci. 12, 1029–1034 (2010)
D. Yamaguchi, M. Hara, Solid State Sci. 12, 1018–1023 (2010)
K. Nakajima, M. Hara, ACS Catal. 2, 1296–1304 (2012)
P.A. Russo, M.M. Antunes, P. Neves, P.V. Wiper, E. Fazio, F. Neri, F. Barreca, L. Mafra, M. Pillinger, N. Pinna, A.A. Valente, Green Chem. 16, 4292–4305 (2014)
P. Gupta, S. Paul, Green Chem. 13, 2365–2372 (2011)
T. Yamazaki, K.Y. Numani, M. Goodman, Biopolymers 31, 1513–1528 (1991)
C.A.G.N. Montalbeti, V. Falque, Tetrahedron 61, 10827–10852 (2005)
J.R. Satam, R.V. Jayaram, Catal. Commun. 9, 2365–2370 (2008)
F. Tamaddon, F. Aboee, A. Nasiri, Catal. Commun. 16, 194–197 (2011)
J.W. Bode, Curr. Opin. Drug Discov. Devel. 9, 765–775 (2006)
H.R. Shaterian, M. Ghashang, M. Feyzi, Appl. Catal. A 345, 128–133 (2008)
T. Dingermann, D. Steinhilber, G. Folkers, Molecular Biology in Medicinal Chemistry, vol. 21 (Wiley-VCH, Weinheim, 2004), pp. 381–398
A.Y. Shen, C.L. Chen, C.I. Lin, Chin. J. Physiol. 35, 45–54 (1992)
F. Al-Assar, K.N. Zelenin, E.E. Lesiovskaya, I.P. Bezhan, B.A. Chakchir, Pharm. Chem. J. 36, 598–603 (2002)
P.V. Pallai, R.S. Struthers, M. Goodman, L. Moroder, E. Wunsch, W. Vale, Biochemistry 24, 1933–1941 (1985)
M. Sechi, U. Azzena, M.P. Delussu, R. Dallocchio, A. Dessi, A. Cosseddu, N. Pala, N. Neamati, Molecules 13, 2442–2461 (2008)
C. Aleman, J. Puiggali, J. Org. Chem. 60, 910–924 (1995)
N.P. Selvam, S. Saranya, P.T. Perumal, Can. J. Chem. 85, 32–38 (2008)
H.R. Sadaati-Mosgtaghin, F.M. Zonoz, M.M. Amini, J. Solid State Chem. 260, 16–22 (2018)
B. Maleki, M. Bhagayeri, RSC Adv. 5, 79746–79758 (2015)
H.A. Soliman, A.Y. Mubarak, S.S. Elmorsy, Chin. Chem. Lett. 27, 353–356 (2016)
R.P. Bakhshani, A. Hassanabadi, J. Chem. Res. 40, 35 (2016)
T.L. Lambat, S.S. Deo, F.S. Inam, T.B. Deshmukh, A.R. Bhat, Karbala Int. J. Mod. Sci. 2, 63–68 (2016)
F.M. Moghaddam, G. Tavakoli, B. Saeednia, Chem. Sel. 2, 1316–1322 (2017)
G. Ramachandran, R. Saraswathi, M. Kumarraja, P. Govindaraj, T. Subramanian, Synth. Commun. 48, 216–222 (2018)
B. Mohammadi, B.R. Khorrami, Montash. Chem. 149, 1089 (2018)
J. Ungar, F. Robinson, J. Pharmacol. Exp. Ther. 80, 217–232 (1944)
I. Antonini, P. Polucci, L.R. Kelland, E. Menta, N. Pescalli, S. Martelli, J. Med. Chem. 42, 2535–2541 (1999)
P.J. McCarthy, T.P. Pitts, G.P. Gunawardana, M. Kelly-Borges, S.A. Pomponi, J. Nat. Prod. 55, 1664–1668 (1992)
D.P. Spalding, E.C. Chapin, H.S. Mosher, J. Org. Chem. 19, 357 (1954)
N. Filloux, J.P. Galy, Synlett 9, 1137–1139 (2001)
I. Antonini, P. Polucci, A. Magnano, D. Cacciamani, M.T. Konieczny, J. Paradziej-Łukowicz, S. Martelli, Bioorg. Med. Chem. 11, 399–405 (2003)
M.A. Pasha, R.R. Khan, K.B. Ramesh, Can. Chem. Trans. 4, 90–98 (2016)
S. Rahmani, A. Amoozadeh, J. Nanostruct. 4, 83–93 (2014)
M. Nasr-Esfahani, M. Montazerozohori, T. Abdizadeh, C. R. Chimie 18, 547–553 (2015)
M. Kiani, M. Mohammadipour, RSC Adv. 7, 997–1007 (2017)
R. Sarada, V. Jagannadharao, B. Govindh, M. Padma, Int. J. Chemtech. Res. 10, 1011–1117 (2017)
A. Jain, S. Singh, K.R. Tiwari, N. Kumar, R. Tomar, Int. J. Mater. Sci. 13, 189–204 (2018)
R. Karmakar, A. Bhaumik, B. Banerjee, C. Mukhopadhyay, Tetrahedron Lett. 58, 622–628 (2017)
H. Sharghi, P. Shiri, M. Aberi, Beilstein J. Org. Chem. 14, 2745–2770 (2018)
K. Nikoofar, F.M. Yielzoleh, J. Saudi Chem. Soc. 22, 715–741 (2018)
M. Mokhtary, Acad. J. Polym. Sci. 2, 555580 (2018)
A. Murugesan, R.M. Gengan, K.G. Moodley, G. Gericke, Adv. Mater. Lett. 8, 773–782 (2017)
H. Singh, H. Singh, A. Singh, M.K. Gupta, S. Sharma, P.M.S. Bedi, Indian J. Pharm. Sci. 79, 801–812 (2017)
S. Mao, F. Li, Y. Lv, C. Lv, S. Yu, Heterocycles 94, 1895–1902 (2017)
F. Hatamjafari, O.H. Lazarjani, Rev. Roum. Chim. 62, 255–260 (2017)
G.J. Bennett, H.H. Lee, Phytochemistry 28, 967–998 (1989)
Y. Na, J. Pharm. Pharmacol. 61, 707–712 (2009)
A.N. Dadhania, V.K. Patel, D.K. Raval, J. Saudi Chem. Soc. 21, S163–S169 (2017)
S. Samantaray, P. Kar, G. Hota, B.G. Mishra, Ind. Eng. Chem. Res. 52, 5862–5870 (2013)
O. Sirkecioglu, N. Talinli, A. Akar, J. Chem. Res. 86, 502–506 (1995)
A. Banerjee, A.K. Mukherjee, Stain Technol. 56, 83–85 (1981)
J. Liu, Z. Diwu, W.Y. Leung, Bioorg. Med. Chem. Lett. 11, 2903–2905 (2001)
G. Song, B. Wang, H. Luo, L. Yang, Catal. Commun. 8, 673–676 (2007)
S. Kantevari, R. Bantu, L. Nagarapu, Arkivoc 16, 136–148 (2006)
F. Nemati, S. Sabaqian, J. Saudi Chem. Soc. 21, S383–S393 (2017)
S. Maripi, R.B. Korupolu, S.B. Madasu, GSC 7, 70–84 (2017)
A.B. Waghamare, S.S. Deshmukh, G.M. Bondle, A.V. Chate, Chem. Biol. Interact. 8, 151–153 (2018)
S.V. Deshmukh, G.K. Kadam, S.V. Shisodia, M.V. Katarina, S.B. Vbale, R.P. Pawar, Int. J. Phys. Chem. Sci. 7, 75 (2018)
B.M. Sapkal, A.J. Sahani, A.S. Burange, S. Kale, G. Abraham, S. Disale, Curr. Catal. 7, 144 (2018)
F.N. Sadeh, M. Fatehpour, N. Hazeri, M.T. Maghsoodlou, M. Lashkari, Acta Chem. Iasi 25, 24 (2017)
S. Karhale, M. Patil, G. Rashinkar, V. Helavi, Res. Chem. Intermed. 43, 7073–7086 (2017)
M. Pirouzmand, A.M. Gharehbaba, Z. Ghasemi, S.A. Khaaje, Arab. J. Chem. 10, 1070 (2017)
R.R. Magar, G.T. Pawar, S.P. Gadekar, M.K. Lande, Bull. Chem. React. Eng. Catal. 13, 436–446 (2018)
M.B. Swami, A.H. Jadhav, N.V. Ghule, S.S. Mahurkar, S.R. Mathapati, A.N. Patil, S.G. Patil, IJGHC 7, 788 (2018)
B. Karami, K. Eskandari, G. Ansari, Der. Chemica. Sinica. 8, 342 (2017)
S.B. Pore, Asian J. Chem. 30, 2621–2624 (2018)
S.U. Deshmukh, G.K. Kadam, S.U. Shisodia, M.V. Katarina, S.B. Ubale, R.P. Pawar, IJCPS 7, 1 (2018)
S.M. Vahdat, M. Akbari, Orient. J. Chem. 24, 1573–1580 (2011)
B. Aday, Y. Yildiz, R. Ulus, S. Eris, F. Sen, M. Kaya, New J. Chem. 40, 748–754 (2016)
Y.B. Shen, G.W. Wang, Arkivoc 16, 1–8 (2008)
B. Das, P. Thirupathi, I. Mahender, V.S. Reddy, Y.K. Rao, J. Mol. Catal. A Chem. 247, 233–239 (2006)
S. Kumari, A. Shekhar, D. Pathak, Chem. Sci. Trans. 3, 652–663 (2014)
G.M. Sheldrick, Acta Cryst. A 64, 112–122 (2008)
G. Ramachandran, R. Saraswathi, M. Kumarraja, P. Govindaraj, T. Subramanian, Synth. Commun. 48, 216–222 (2018)
N. Azizi, M. Alipour, J. Mol. Liq. 206, 268–271 (2015)
H.A. Soliman, A.Y. Mubarak, S.S. Elmorsy, Chin. Chem. Lett. 27, 353–356 (2016)
S. Khabnadideh, K. Zomorodian, B.B.F. Mirjalili, E. Izadi, L. Zamani, J. Chil. Chem. Soc. 61, 3116 (2016)
K. Selvakumar, T. Shanmugaprabha, M. Kumaresan, P. Sami, Synth. Commun. 47, 2115–2126 (2017)
A. Khojastehnezhad, F. Moeinpour, M. Vafaei, J. Mex. Chem. Soc. 59, 29–35 (2015)
A.P. Marjani, J. Khalafy, S. Mahmoodi, ARKIVOC iii, 262–270 (2016)
S.-J. Yü, S. Wu, X.-M. Zhao, C.-W. Lü, Res. Chem. Intermed. 43, 3121–3130 (2017)
G.D. Shirole, S. Bhalekar, S.N. Shelke, Indian J. Chem. 57B, 1430–1435 (2018)
M.A. Mangalavathi, J. Pasha, Chem. Chem. Sci. 8, 1009–1017 (2018)
M. Pirouzmand, A.M. Gharehbaba, Z. Ghasemi, S.A. Khaaje, Arab. J. Chem. 10, 1070–1076 (2017)
F. Nemati, S. Sabaqian, J. Saudi Chem. Soc. 21, S383–S393 (2017)
S. Maripi, R.B. Korupolu, S.B. Madasu, Green Sustain. Chem. 7, 70–84 (2017)
A. Thakur, A. Sharma, A. Sharma, Synth. Commun. 46, 1766–1771 (2016)
R. Khoeiniha, A. Ezabadia, A. Olyaei, Iran. Chem. Commun. 4, 273–282 (2016)
K. Hemalatha, G. Madhumitha, A. Kajbafvala, N. Anupama, R. Sompalle, S.M. Roopan, J. Nanomater. 2013, 1–23 (2013)
S. Erdem, B. Erdem, R.M. Oksuzoglu, Open Chem. 16, 923–929 (2018)
F.D. Guerra, M.F. Attia, D.C. Whitehead, F. Alexis, Molecules 23, 1760 (2018)
P. Singh, M. Abdullah, S. Ikram, Nano Res. Appl. 2, 1–10 (2016)
M.M. Khin, A.S. Nair, V.J. Babu, R. Murugana, S. Ramakrishna, Energy Environ. Sci. 5, 8075–8109 (2012)
Acknowledgements
I am grateful to Director, SAIF, IIT Bombay for SEM, TEM. I also extend my thanks to Prof. R. K. Bamezai, Department of Chemistry, University of Jammu for recording TGA. I am thankful to Prof. H. N. Sheikh for providing me the FTIR analysis. We are also thankful to the department of Physics for providing us the XRD data of the crystal and Dr. Amit Kumar Sharma for providing me the NMR data of the prepared compounds.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kour, J., Gupta, M., Chowhan, B. et al. Carbon-based nanocatalyst: An efficient and recyclable heterogeneous catalyst for one-pot synthesis of gem-bisamides, hexahydroacridine-1,8-diones and 1,8-dioxo-octahydroxanthenes. J IRAN CHEM SOC 16, 2587–2612 (2019). https://doi.org/10.1007/s13738-019-01723-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01723-1




