Abstract
Good to excellent yields of α-aminonitriles are achieved through three-component Strecker reaction of aldehydes or ketones with amines and trimethylsilyl cyanides over homopiperazine sulfamic acid functionalized mesoporous silica nanoparticles (MSNs-HPZ-SO3H). The advantages of this protocol include: simplicity, short reaction time, high yields, ease of product isolation and reusability of the catalyst.
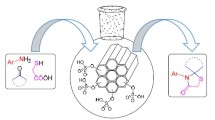
Graphical abstract
MSNs-HPZ-SO3H is prepared as an acid catalyst and successfully used for three-component Strecker reaction of aldehydes or ketones with amines and trimethylsilyl cyanides (TMSCN) under solvent-free conditions, a straightforward strategy for the synthesis of α-aminonitriles.





Similar content being viewed by others
References
L. Banfi, G. Guanti, R. Riva, Chem. Commun. 11, 985 (2000)
W.L. Matier, D.A. Owens, W.T. Comer, D. Deitchman, H.C. Ferguson, R.J. Seidehamel, J.R. Young, J. Med. Chem. 16, 901 (1973)
J. Marco, S.T. Ingate, P. Manzano, Tetrahedron Lett. 39, 4123 (1998)
S. Velázquez, C. Chamorro, M.-J. Pérez-Pérez, R. Alvarez, M.-L. Jimeno, A. Martín-Domenech, C. Pérez, F. Gago, E. De Clercq, J. Balzarini, J. Med. Chem. 41, 4636 (1998)
D. Enders, J.P. Shilvock, Chem. Soc. Rev. 29, 359 (2000)
R.O. Duthaler, Tetrahedron 50, 1539 (1994)
S.J. Zuend, M.P. Coughlin, M.P. Lalonde, E.N. Jacobsen, Nature 461, 968 (2009)
L.M. Weinstock, P. Davis, B. Handelsman, R.J. Tull, J. Org. Chem. 32, 2823 (1967)
A. Strecker, Liebigs Ann. Chem. 75, 27 (1850)
B.A.B. Prasad, A. Bisai, V.K. Singh, Tetrahedron Lett. 45, 9565 (2004)
S.K. De, R.A. Gibbs, J. Mol. Catal. A: Chem. 232, 123 (2005)
S. Kobayashi, T. Busujima, Chem. Commun. 9, 981 (1998)
S.K. De, J. Mol. Catal. A: Chem. 225, 169 (2005)
Z.-L. Shen, S.-J. Ji, T.-P. Loh, Tetrahedron 64, 8159 (2008)
A. Majhi, S.S. Kim, S.T. Kadam, Tetrahedron 64, 5509 (2008)
M. Narasimhulu, T.S. Reddy, K.C. Mahesh, S.M. Reddy, A.V. Reddy, Y. Venkateswarlu, J. Mol. Catal. A: Chem. 264, 288 (2007)
B. Karimi, A.A. Safari, J. Organomet. Chem. 693, 2967 (2008)
B.M. Reddy, B. Thirupathi, M.K. Patil, J. Mol. Catal. A: Chem. 307, 154 (2009)
J.S. Yadav, B.V.S. Reddy, B. Eshwaraiah, M. Srinivas, P. Vishnumurthy, New J. Chem. 27, 462 (2003)
M.Z. Kassaee, H. Masrouri, F. Movahedi, Appl. Catal. A Gen. 395, 28 (2011)
S. Baghery, M.A. Zolfigol, R. Schirhagl, M. Hasani, M.C.A. Stuart, A. Nagl, Appl. Organomet. Chem. 31, e3883 (2017)
B. Karimi, D. Zareyee, J. Mater. Chem. 19, 8665 (2009)
J. Jarusiewicz, Y. Choe, K.S. Yoo, C.P. Park, K.W. Jung, J. Org. Chem. 74, 2873 (2009)
K. Iwanami, H. Seo, J.-C. Choi, T. Sakakura, H. Yasuda, Tetrahedron 66, 1898 (2010)
A. Heydari, A. Arefi, S. Khaksar, R.K. Shiroodi, J. Mol. Catal. A: Chem. 271, 142 (2007)
B. Karmakar, J. Banerji, Tetrahedron Lett. 51, 2748 (2010)
M.G. Dekamin, M. Azimoshan, L. Ramezani, Green Chem. 15, 811 (2013)
K. Surendra, N.S. Krishnaveni, A. Mahesh, K.R. Rao, J. Org. Chem. 71, 2532 (2006)
J. Wang, Y. Masui, M. Onaka, Eur. J. Org. Chem. 2010, 1763 (2010)
F. Rajabi, S. Nourian, S. Ghiassian, A.M. Balu, M.R. Saidi, J.C. Serrano-Ruiz, R. Luque, Green Chem. 13, 3282 (2011)
M. Shekouhy, Catal. Sci. Technol. 2, 1010 (2012)
S. Baghery, M.A. Zolfigol, M. Safaiee, D.A. Alonso, A. Khoshnood, Appl. Organomet. Chem. 31, e3775 (2017)
J.S. Yadav, B.V.S. Reddy, B. Eeshwaraiah, M. Srinivas, Tetrahedron 60, 1767 (2004)
A. Dutta, J. Mondal, A.K. Patra, A. Bhaumik, Chem. Eur. J. 18, 13372 (2012)
K. Ghosh, R.A. Molla, M.A. Iqubal, S.M. Islam, Green Chem. 17, 3540 (2015)
M. Hajjami, F. Ghorbani, F. Bakhti, Appl. Catal. A Gen. 470, 303 (2014)
Z. Nasresfahani, M.Z. Kassaee, E. Eidi, New J. Chem. 40, 4720 (2016)
I.I. Slowing, B.G. Trewyn, V.S.-Y. Lin, J. Am. Chem. Soc. 129, 8845 (2007)
H. Ghafuri, A. Rashidizadeh, B. Ghorbani, M. Talebi, New J. Chem. 39, 4821 (2015)
H. Singh, J.K. Rajput, P. Arora, RSC Adv. 6, 84658 (2016)
B.C. Ranu, S.S. Dey, A. Hajra, Tetrahedron 58, 2529 (2002)
G.K.S. Prakash, E. Thomas, I. Bychinskaya, A.G. Prakash, C. Panja, G.A. Olah, Green Chem. 10, 1105 (2008)
J.G. Hernández, M. Turberg, I. Schiffers, C. Bolm, Chem. Eur. J. 22, 14513 (2016)
A. Mobaraki, B. Movassagh, B. Karimi, ACS Comb. Sci. 16, 352 (2014)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Mohamad Z. Kassaee: Visiting Scholar (sabbatical).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nasresfahani, Z., Kassaee, M.Z. & Eidi, E. Efficient synthesis of α-aminonitriles over homopiperazine sulfamic acid functionalized mesoporous silica nanoparticles (MSNs-HPZ-SO3H), as a reusable acid catalyst. J IRAN CHEM SOC 16, 1819–1825 (2019). https://doi.org/10.1007/s13738-019-01654-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01654-x