Abstract
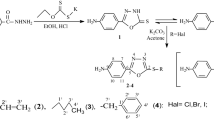
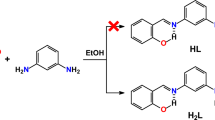
Three pyrazole Schiff bases (E)-2-(((1H-pyrazol-3-yl)imino)methyl)-6-methoxyphenol (1), (Z)-N-(4-bromobenzylidene)-1H-pyrazol-3-amine (2), and (E)-2-(((1H-pyrazol-3-yl)imino) methyl)-4,6-dibromophenol (3) have been synthesized and characterized by elemental analyses, FT-IR, 1HNMR. The molecular structures were confirmed by X-ray structural studies, and investigated intermolecular interactions in building different supramolecular architectures. The title compounds are associated through hydrogen bonds, π-stacking interactions and further connected into hydrogen-bonded supramolecular layers and are responsible as well for the strengthening of the molecular assembly. In addition, the title compounds were also tested for their ability to inhibit the growth of C. albicans and Gram-negative bacteria. It was worthwhile to note that 1 and 3 could be used as potential antibacterial agents.




Similar content being viewed by others
References
C.B. Aakeröy, N.R. Champnes, C. Janiak, CrystEngComm 12, 22 (2010)
D. Braga, L. Brammer, N.R. Champness, CrystEngComm 7, 1 (2005)
M. Dinca, J.R. Long, Angew. Chem., Int. Ed. 47, 6766 (2008)
A.I. Kitaigorodskii, Molecular Crystals and Molecules (Academic Press, New York, 1973)
G.R. Desiraju, J.A.R.P. Sarma, Proc. Indian Acad. Sci. Chem. Sci. 96, 599 (1986)
F.H. Allen, Acta Crystallogr. Sect. B Struct. Sci. 58, 380 (2002)
M. Polito, E. D’Oria, L. Maini, P.G. Karamertzanis, F. Grepioni, D. Braga, S.L. Price, CrystEngComm 10, 1848 (2008)
C. Janiak. J. Chem. Soc. Dalton Trans. 21, 3885–3896 (2000)
H. Zhu, M. Ströbele, Z. Yu, Z. Wang, H.-J. Meyer, X. You, Inorg. Chem. Commun. 4, 577–581 (2001)
M.J. MacLachlan, Pure Appl. Chem. 78, 873–888 (2006)
Y.R. Li, C. Li, J.C. Liu, M. Guo, T.Y. Zhang, L.P. Sun, C.J. Zheng, H.R. Piao, Bioorg. Med. Chem. Lett. 25, 5052 (2015)
J.X. Wang, Z.R. Zhu, F.Y. Bai, X.Y. Wang, X.X. Zhang, Y.H. Xing, Polyhedron 99, 59 (2015)
E. Akbas, I. Berber, A. Sener, B. Hasanov, Il Farmaco 60, 23 (2005)
G.M. Sheldrick, SHELXL97, Program for the refinement of crystal structures (University of Göttingen, Germany, 1997)
H.D. Flack, Acta Cryst. A39, 876 (1983)
A.C. Cunha, V.F. Ferreira, A.K. Jordão, M.C.B.V. de Souza, S.M.S.V. Wardell, J.L. Wardell, P.A. Tan, R.P.A. Bettens, S.K. Seth, E. R. T. Tiekink, CrystEngComm 15, 4917 (2013)
S.K. Seth, D. Sarkar, A. Roy, T. Kar, CrystEngComm 13, 6728–6741 (2011)
S.K. Seth, D. Sarkar, T. Kar, CrystEngComm 13, 4528–4535 (2011)
S.K. Seth, D. Sarkar, A.D. Jana, T. Kar, Cryst. Growth Des. 11, 4837–4849 (2011)
J. Bernstein, R.E. Davis, L. Shimoni, N.L. Chang, Angew. Chem. Int. Ed. 34, 1555 (1995)
I. Berber, C. Cokmus, E. Atalan, Microbiology 72, 54 (2003)
L.A. Mitscher, S.P. Pillai, E.J. Gentry, D.M. Shankel, Med. Res. Rev. 19, 477 (1999)
V. Lee, S.J. Hecker, Med. Res. Rev. 19, 521 (1999)
E.V. Pimerova, E.V. Voronina, Pharm. Chem. J. 35, 18 (2001)
V.A. Chornous, M.K. Bratenko, M.V. Vovk, I.I. Sidorchuk, Pharm. Chem. J. 35, 26 (2001)
Acknowledgements
We gratefully acknowledge the Key Research Project of Natural Science from Provincial Bureau of Education, Anhui, China (KJ2017A572), the National Natural Science Foundation of China (20801012), and the financial support from Jiangsu Ainaji Neoenergy Science and Technology Co., Ltd (8507040091).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Feng, C., Guo, JJ., Sun, LN. et al. Pyrazole Schiff bases cross-linked supramolecules: structural elucidation and antibacterial activity. J IRAN CHEM SOC 15, 2871–2876 (2018). https://doi.org/10.1007/s13738-018-1473-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-018-1473-1