Abstract
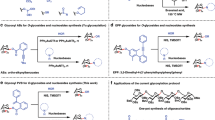
An efficient and rapid regiospecific approach for the synthesis of cyclic and acyclic nucleosides of 2-oxonicotinonitriles was performed. Whereas, glycosylation of 2-oxonicotinonitriles 1a, b with peracetylated sugars (namely, peracetylated glucose, galactose and ribose) under MWI tolerated exclusively the desired N-nucleosides 2a, b, 4a, b and 6a, b in significant yields (75–86%) and in short reaction time (5–7 min.). The same products were obtained under the conventional conditions, using halo-sugar with low yields in hard conditions. Similarly, the acyclic nucleosides 8a, b and 9a, b were obtained under MWI and conventional conditions via reaction of 1a, b with 4-bromo butyl acetate and 2-acetoxyethoxymethyl bromide. Finally, deprotection of the latter blocked N-nucleosides was performed via treatment with aqueous methanolic solution containing a catalytic amount of triethyl amine to give the desired free nucleosides 3a, b, 5a, b, 7a, b, 10a, b and 11a, b, respectively. The free nucleosides (3a, b, 5a, b, 7a, b and 11a, b) were evaluated against Gram (+ ve) bacteria, Gram (–ve) bacteria and one pathogenic Fungi namely, Aspergillus flavus. Good results were obtained for compounds 7a, b and 11a, b compared with the used standard drugs (Cefotaxime and Dermatin).
Graphical abstract



Similar content being viewed by others
References
A.H. Abadi, T.M. Ibrahim, K.M. Abouzid, J. Lehmann, H.N. Tinsley, B.D. Gary, G.A. Piazza, Bioorg. Med. Chem. 5974, 17 (2009)
O.M. Abdelhafez, K.M. Amin, R.Z. Batran, T.J. Maher, S.A. Nada, S. Sethumadhavan, Bioorg. Med. Chem. 3371, 18 (2010)
A.G. Hammam, N.A. Abdel Hafez, W.H. Midura, M.Z. Mikolajczyk, Z. Naturforsch. 417, 55 (2000)
M.M. Ismail, Y.A. Ammar, H.S. El-Zahaby, S.I. Eisa, E.S. Barakat, Arch. Pharm. 476, 340 (2007)
M.A. Gouda, M.A. Barghot, G.E. Abd El-Ghani, A.M. Khalil, J. Heterocyclic Chem. 1241, 53 (2016)
N. Siddiqui, W. Ahsan, M.S. Alam, R. Ali, K. Srivastava, Arch. Pharm. Chem. Life Sci. 185, 345 (2012)
H.A. El-Sayed, A.H. Moustafa, A.E. El-Torky, E.A. Abd El-Salam, Russ. J. Gen. Chem. 2401, 87 (2017)
S.A. Said, A.E. Amr, H.A. El-Sayed, M.A. Al-Omar, M.M. Abdalla, Int. J. Pharmacol 502, 11 (2015)
H.A. El-Sayed, A.H. Moustafa, A.Z. Haikal, R. Abu-El-Halawa, E.H. El, Ashry, Eur. J. Med. Chem. 2948, 46 (2011)
A.H. Moustafa, H.A. El-Sayed, A.Z. Haikal, R.A. Abd El-Hady, Nucleosides Nucleotides Nucleic Acids 221, 32 (2013)
R.A. Haggam, H.A. El-Sayed, S.A. Said, M.H.M. Ahmed, A.H. Moustafa, R.E. Abd-El-Noor, J. Heterocyclic Chem. 375, 54 (2017)
R.A.I. Abou-Elkhair, A.H. Moustafa, A.Z. Haikal, A.M. Ibraheem, Eur. J. Med. Chem. 388, 74 (2014)
A.E. Rashad, A.H. Shamroukh, M.A. El-Hashash, A.F. El-Farargy, N.M. Yousif, M.A. Salama, A. Mostafa, M. El-Shahat, J. Heterocyclic Chem. 1130, 49 (2012)
B.A.Hussain,A.M.Attia, G.E.H. Elgemeie, Nucleosides Nucleotides 2335, 18 (1999)
S.S. Al-Neyadi, A.H. Hassan, I.M. Abdou, Nucleosides Nucleotides Nucleic Acids 120, 30 (2011)
L. Strekowski, I.M. Abdou, A.M.E. Attia, S.E. Patterson, Tetrahed. Lett. 4757, 41 (2000)
A.E. Rashad, A.H. Shamroukh, H.H. Sayed, S.M. Awad, N.A.M. Abdelwahed, Synth. Commun. 652, 41 (2011)
N.A. Al-Masoudi, B.A. Saeed, A.H. Essa, Y.A. Al-Soud, Nucleosides Nucleotides Nucleic Acids 175, 28 (2009)
H.A. El-Sayed, N.H. Ouf, A.H. Moustafa, Res. Chem. Intermed. 407, 40 (2014)
R.A. Haggam, H.A. El-Sayed, Res. Chem. Intermed. 8159, 41 (2015)
National committee for clinical laboratory standards (NCCLS), Methods for dilution antimicrobial susceptibility tests of bacteria that grow aerobically”; Approved standard M100-512 (P. A. Wayne, NCCLS, 2002)
Acknowledgements
The authors are grateful to the stuff member of microbiology Lab., Faculty of Science, Zagazig University for performance the antimicrobial activity and organic chemistry research lab for carrying out the all experiment part.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moustafa, A.H., Ahmed, M.H.M. Microwave in glycosylation reaction: design, and synthesis of highly substituted nicotinonitrile nucleosides. J IRAN CHEM SOC 15, 2107–2115 (2018). https://doi.org/10.1007/s13738-018-1403-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-018-1403-2