Abstract
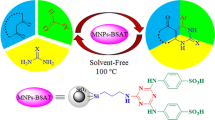
4-(4-Propylpiperazine-1-yl)butane-1-sulfonic acid-modified silica-coated magnetic nanoparticles have been synthesized via covalent grafting of piperazine on (3-chloropropyl) triethoxysilane-functionalized magnetic nanoparticles through ring opening reaction of 1,4-butane sultone. The prepared reagent was characterized using different types of methods, including infrared spectroscopy (FT-IR), X-ray diffraction, thermogravimetric analysis, and scanning electron microscopy. After characterization, the catalytic activity of this reagent in the preparation of 1-(benzothiazolylamino) phenylmethyl-2-naphthols via one-pot condensation of aldehydes, 2-aminobenzothiazole, and 2-naphthol was studied in the absence of solvent. All reactions were performed under mild conditions in excellent yields during short reaction times. Using this method, the catalyst can be easily isolated from the reaction mixture by magnetic decantation using an external magnet and reused at least seven times without significant degradation in its activity.









Similar content being viewed by others
References
S. Sobhani, Z. Pakdin Parizi, N. Razavi, Appl. Catal. A Gen. 409–410, 162 (2011)
S. Mornet, S. Vasseur, F. Grasset, E. Duguet, J. Mater. Chem. 14, 2161 (2004)
M.F. Casula, A. Corrias, P. Arosio, A. Lascialfari, T. Sen, P. Floris, I.J. Bruce, J. Colloid Interface Sci. 357, 50 (2011)
J.D.G. Duran, J.L. Arias, V. Gallardo, A.V. Delgado, J. Pharm. Sci. 97, 2948 (2008)
A.H. Latham, M.E. Williams, Acc. Chem. Res. 41, 411 (2008)
T. Zeng, W.W. Chen, C.M. Cirtiu, A. Moores, C.J. Song, G. Li, Green Chem. 12, 570 (2010)
M.B. Gawande, A.K. Rathi, I.D. Nogueira, R.S. Varma, P.S. Branco, Green Chem. 15, 1895 (2013)
P. Selvam, in Encyclopedia of Nanoscience and Nanotechnology, vol. 6, 2nd edn., ed. by H.S. Nalwa (American Scientific Publishers, Los Angeles, 2004), p. 215
S.Z. Hejazi, A.F. Shojaei, K. Tabatabaeian, F. Shirini, J. Serb. Chem. Soc. 80, 971 (2015)
M.Z. Kassaee, H. Masrouri, F. Movahedi, Appl. Catal. A Gen. 395, 28 (2011)
A. Kong, P. Wang, H. Zhang, F. Yang, S.P. Huang, Y. Shan, Appl. Catal. A Gen. 183, 417 (2012)
M.M. Mojtahedi, M.S. Abaee, T. Alishiri, Tetrahedron Lett. 50, 2322 (2009)
M. Seddighi, F. Shirini, M. Mamaghani, C. R. Chim. 18, 573 (2015)
A. Catalano, A. Carocci, I. Defrenza, M. Muraglia, A. Carrieri, F.V. Bambeke, A. Rosato, F. Corbo, C. Franchini, Eur. J. Med. Chem. 64, 357 (2013)
R.V. Patel, P.K. Patel, P. Kumari, D.P. Rajani, K.H. Chikhalia, Eur. J. Med. Chem. 53, 41 (2012)
D. Fajkusova, M. Pesko, S. Keltosova, J. Guo, Z. Oktabec, M. Vejsova, P. Kollar, A. Coffey, J.C. Sollei, K. Kralova, J. Jampilek, Bioorg. Med. Chem. 20, 7059 (2012)
V.N. Telveka, V.K. Bairwa, K. Satardekar, A. Bellubi, Bioorg. Med. Chem. Lett. 22, 649 (2012)
G. Alang, R. Kaur, G. Kaur, A. Singh, P. Singla, Acta Pharm. Sci. 52, 213 (2010)
S.N. Manjula, N.M. Noolvi, K.V.M. Parihar, S.A. Reddy, V. Ramani, A.K. Gadad, G. Singh, N.G. Kutty, C.M. Rao, Eur. J. Med. Chem. 44, 2923 (2009)
H.R. Shaterian, A. Hosseinian, Res. Chem. Intermed. 41, 793 (2015)
S. Javanshir, A. Ohanian, M.M. Heravi, M.R. Naimi-Jamal, F.F. Bamoharram, J. Saudi Chem. Soc. 18, 502 (2014)
A. Shaabani, A. Rahmati, E. Farhangi, Tetrahedron Lett. 48, 7291 (2007)
Y. Yi, G. Hongyunv, Chin. J. Org. Chem. 31, 96 (2011)
M.H. Valkenberg, C. deCastro, W.F. Holderich, Green Chem. 4, 88 (2002)
A. Hosseinian, H.R. Shaterian, Phosphorus, Sulfur Silicon Relat. Elem. 187, 1056 (2012)
L. Yang, Eur. J. Chem. 9, 2424 (2012)
Z. Cheng, Z. Gao, W. Maa, Q. Sun, B. Wang, X. Wang, Chem. Eng. J. 209, 451 (2012)
F. Shirini, M. Abedini, M. Seddighi, J. Nanosci. Nanotechnol. 16, 8208 (2016)
N. Seyyedi, F. Shirini, M. Safarpoor Nikoo Langarudi, RSC Adv. 6, 44630 (2016)
O. Goli-Jolodar, F. Shirini, M. Seddighi, J. Iran. Chem. Soc. 13, 475 (2016)
F. Shirini, M. Mazloumi, M. Seddighi, Res. Chem. Intermed. 42, 1759 (2016)
F. Shirini, M. Abedini, S. Zarrabzadeh, M. Seddighi, J. Iran. Chem. Soc. 12, 2105 (2015)
F. Shirini, N. Daneshvar, RSC Adv. 6, 110190 (2016)
S. Sobhani, M.S. Ghasemzadeh, M. Honarmand, Catal. Lett. 144, 1515 (2014)
J. Wang, S. Zheng, Y. Shao, J. Liu, Z. Xu, D. Zhu, J. Colloid Interface Sci. 349, 293 (2010)
M.N. Sefat, D. Saberi, K. Niknam, Catal. Lett. 141, 1713 (2011)
O.G. Jolodar, F. Shirini, M. Seddighi, RSC Adv. 6, 26026 (2016)
H.N. Dadhania, D.K. Ravala, A.N. Dadhania, RSC Adv. 5, 4806 (2015)
A. Kumar, M.S. Rao, V.K. Rao, Aust. J. Chem. 63, 1538 (2010)
H.R. Shaterian, A. Hosseinian, Sci. Iran. C 21, 727 (2014)
P.K. Kalavagunta, P.K. Bagul, A. Jallapally, S. Kantevari, S.K. Banerjee, N. Ravirala, Eur. J. Med. Chem. 83, 344 (2014)
M.A. Zolfigol, M. Navazeni, M. Yarie, R. Ayazi-Nasrabadi, Res. Chem. Intermed. 44, 191 (2018)
F. Kamali, F. Shirini, Appl. Organomet. Chem. 42, 3972 (2017)
Acknowledgements
The authors are thankful to the Guilan and Zanjan Universities Research Councils for the partial support of this work.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lati, M.P., Shirini, F., Alinia-Asli, M. et al. Synthesis of 1-(benzothiazolylamino)phenylmethyl-2-naphthols accelerated by a novel magnetic nanocatalyst. J IRAN CHEM SOC 15, 1655–1662 (2018). https://doi.org/10.1007/s13738-018-1364-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-018-1364-5