Abstract
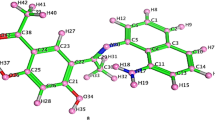
A novel Schiff base, 3-(((1H-1,2,4-triazol-3-yl)imino)methyl)-4H-chromen-4-one (L) was synthesized and used as ligand for the synthesis of Co(II), Ni(II), Cu(II), Zn(II) and Pd(II) complexes. The structural characterization of the ligand and its metal complexes was determined by using various physicochemical and spectroscopic methods. The IR data show that the Schiff base ligand acts as a bidentate donor coordinating through the oxygen atom of the chromone and nitrogen atom of the imine group. Based on all spectral data, tetrahedral geometry has been proposed for all the metal complexes except Cu(II) and Pd(II) complexes. However, square-planar geometry has been proposed for Cu(II) and Pd(II) complexes. DNA binding interaction of the ligand and its metal complexes was investigated by using UV–visible absorption, fluorescence and molecular docking studies. The binding constants were in the order of 104 M−1 suggesting good binding affinity towards CT-DNA. The DNA cleavage activity of the synthesized compounds was investigated by using agarose gel electrophoresis. In vitro antimicrobial activity of the synthesized compounds were screened against two gram-positive bacteria (Bacillus subtilis, Staphylococcus aureu) and two gram-negative bacteria (Escherichia coli, Proteus vulgaris) and one fungi strain Candida albicans using disc diffusion method. Antioxidant activity was carried out by DPPH radical scavenging method. In vitro anti-proliferative activity of the ligand and its metal complexes was also carried on the HEK-293, HeLa, IMR-32 and MCF-7 cancer cell lines using MTT assay.












Similar content being viewed by others
References
A.A.M. Belal, I.M. El-Deen, N.Y. Farid, R. Zakaria, M.S. Refat, Spectrochim. Acta A 149, 771 (2015)
M. Salehi, M. Amirnasr, S. Meghdadi, K. Mereiter, H.R. Bijanzadeh, A. Khaleghian, Polyhedron 81, 90 (2014)
S. Esmaielzadeh, L. Azimian, K. Shekoohi, H. Esfandiari, M. Asadi, Z. Zare, A. Rahmani Nejad, K. Mohammadi, Inorg. Chim. Acta 405, 155 (2013)
M. Muthu Tamizh, R. Karvembu, Inorg. Chem. Commun. 25, 30 (2012)
P. Mendu, C.G. Kumari, R. Ragi, J. Fluoresc. 25, 369 (2015)
M. Montazerozohori, S.A. Musavi, A. Masoudiasl, A. Naghiha, M. Dusek, M. Kucerakova, Spectrochim. Acta A 137, 389 (2015)
V.Y. Sasnovskikh, R.A. Irgashev, Tetrahedron Lett. 48, 7436 (2007)
Z. Siddiqui, F. Farooq, J. Chem. Sci. 124, 1097 (2012)
O.A. El-Gammal, G.A. El-Reash, S.F. Ahmed, J. Mol. Struct. 1007, 1 (2012)
L. Puccetti, G. Fasolis, D. Vullo, Z.H. Chohan, A. Scozzafava, C.T. Supuran, Bioorg. Med. Chem. Lett. 15, 3096 (2005)
J. Nawrot-Modranka, E. Nawrot, Acta Pol. Pharm. 63, 429 (2007)
C. Anitha, C.D. Sheela, P. Tharmaraj, S. Johnson Raja, Spectrochim. Acta A 98, 35 (2012)
J. Wang, Z.Y. Yang, X.Y. Yi, B.D. Wang, J. Photochem. Photobiol., A 201, 183 (2009)
S.A. Elsayed, I.S. Butler, B.J. Claude, S.I. Mostafa, Transit. Metal Chem. 40, 179 (2015)
Y. Li, Z. Yang, J. Coord. Chem. 63, 1960 (2010)
M. Grazul, E. Budzisz, Coordin. Chem. Rev. 253, 2588 (2009)
Y. Li, Z.Y. Yang, J. Fluoresc. 20, 329 (2010)
J. Wang, Z.Y. Yang, B.D. Wang, X.Y. Yi, Y.C. Liu, J. Fluoresc. 19, 847 (2009)
B.D. Wang, Z.Y. Yang, D.W. Zhang, Y. Wang, Spectrochim. Acta A 63, 213 (2006)
D.D. Qin, G.F. Qi, Z.Y. Yang, J.C. Wu, Y.C. Liu, J. Fluoresc. 19, 409 (2009)
B.D. Wang, Z.Y. Yang, P. Crewdson, D.Q. Wang, J. Inorg. Biochem. 101, 1492 (2007)
K. Mansouri, R. Khodarahmi, A. Foroumadi, A. Mostafaie, H.M. Motlagh, Med. Chem. Res. 20, 920 (2011)
S.X. Cai, J. Drewe, W. Kemnitzer, Anticancer Agents Med. Chem. 9, 437 (2009)
H. Adibi, R. Khodarahmi, K. Mansouri, M. Khaleghi, S. Maghsoudi, Pharm. Sci. 19, 23 (2013)
Z. Baráth, R. Radics, G. Spengler, I. Ocsovszki, M. Kawase, N. Motohashi, Y. Shirataki, A. Shah, J. Molnár, In Vivo 20, 645 (2006)
P.S. Ishar, G. Singh, S. Singh, K.K. Sreenivasan, G. Singh, Bioorganic Med. Chem. Lett. 16, 1366 (2006)
A.M.A. Hassan, A.I. Hanafy, M.M. Ali, A.A. Salman, Z.A. El-Shafay, Z.H. Abd El-Wahab, I.A. Salama, J. Basic Appl. Chem. 2, 1 (2012)
A.K. Bishnoi, R. Dass, R.G. Sharma, Anal. Sci. 20, 921 (2004)
M. Kalanithi, D. Kodimunthiri, M. Rajarajan, P. Tharmaraj, Spectrochim. Acta A 82, 290 (2011)
P. Kavitha, M. Saritha, K. Laxma Reddy, Spectrochim. Acta A 102, 159 (2013)
P. Kavitha, K. Laxma Reddy, Bioinorg. Chem. Appl. 2014 (2014)
P. Kavitha, K. Laxma Reddy, Arab. J. Chem. 9, 596 (2016)
P. Kavitha, K. Laxma Reddy, Arab. J. Chem. 9, 640 (2016)
P. Kavitha, M.R. Chary, B.V.A.A. Singavarapu, K. Laxma Reddy, J. Saudi Chem. Soc. 20, 69 (2016)
V. Barve, F. Ahmed, S. Adsule, S. Banerjee, S. Kulkarni, P. Katiyar, C.E. Anson, A.K. Powell, S. Padhye, F.H. Sarkar, J. Med. Chem. 49, 3800 (2006)
F. Arjmand, F. Sayeed, M. Muddassir, J. Photochem. Photobiol., B 103, 166 (2011)
A.D. Kulaczkowska, J. Therm. Anal. Calorim. 109, 7 (2012)
Y. Li, Z.Y. Yang, ZCh. Liao, Z.C. Han, Z.C. Liu, Inorg. Chem. Commun. 13, 1213 (2010)
Y. Li, Z.Y. Yang, J.C. Wu, Eur. J. Med. Chem. 45, 5692 (2010)
Y. Li, Z.Y. Yang, T.R. Li, Z.C. Liu, B.D. Wang, J. Fluoresc. 21, 1091 (2011)
O. Bekircan, Z. Bıyıklıoglu, I. Acar, H. Bektas, H. Kantekin, J. Organomet. Chem. 693, 3425 (2008)
J. Chen, X.Y. Sun, K.Y. Chai, J.S. Lee, M.S. Song, Z.S. Quan, Med. Chem. 15, 6775 (2007)
O. Bekircan, B. Kahveci, O.B. Özgümüs, Chin. J. Chem. 25, 1871 (2007)
R. Lesyka, O. Vladzimirska, S. Holota, L. Zaprutko, A. Gzella, Eur. J. Med. Chem. 42, 641 (2007)
O. Kahn, C.J. Martinez, Devices Sci. 279, 44 (1998)
Y. Garcia, P.J. Koningsbruggen, E. Codjovi, R. Lapouyeda, O. Kahn, L. Rabardel, J. Mater. Chem. 7, 857 (1997)
A. Nohara, T. Umetani, Y. Sanno, Tetrahedron Lett. 14, 1995 (1973)
N. Vamsikrishna, M. Pradeep Kumar, R. Kumar, G. Ramesh, N. Ganji, S. Daravath, J. Chem. Sci. 129, 609 (2017)
M.R. Efink, C.A. Ghiron, Anal. Biochem. 114, 199 (1981)
E.F. Pettersen, T.D. Goddard, C.C. Huang, G.S. Couch, D.M. Greenblatt, E.C. Meng, J. Comput. Chem. 25, 1605 (2004)
G.M. Morris, D.S. Goodsell, R.S. Halliday, J. Comput. Chem. 19, 1639 (1998)
J. Sambrook, E.F. Fritsch, T. Maniatis, Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, 1989)
N. Shahabadi, S. Kashanian, F. Darabi, Eur. J. Med. Chem. 45, 4239 (2010)
A. Braca, N. de Tommasi, L. di Bari, C. Pizza, M. Politi, I. Morelli, J. Nat. Prod. 64, 892 (2001)
W.J. Geary, Coordin. Chem. Rev. 7, 81 (1971)
H.P. Ebrahimi, J.S. Hadi, Z.A. Abdulnabi, Z. Bolandnazar, Spectrochim. Acta A 117, 485 (2014)
B.D. Wang, Z.Y. Yang, D.D. Qin, Z.N. Chen, J. Photochem. Photobiol., A 194, 49 (2008)
A.K. Singh, O.P. Pandey, S.K. Sengupta, Spectrochim. Acta A 85, 1 (2012)
D. Arish, M. Sivasankaran Nair, Spectrochim. Acta A 82, 191 (2011)
S. Sobha, R. Mahalakshmi, N. Raman, Spectrochim. Acta A 92, 175 (2012)
D.M.A. El-Aziz, S.E.H. Etaiw, E.A. Ali, J. Mol. Struct. 1048, 487 (2013)
S.E.H. Etaiw, D.M.A. El-Aziz, E.H.A. El-Zaher, E.A. Ali, Spectrochim. Acta A 79, 1331 (2011)
C.J. Dhanaraj, M.S. Nair, J. Saudi Chem. Soc. 18, 479 (2014)
S. Chattopadhyay, G. Bocelli, A. Cantoni, A. Ghosh, Inorg. Chim. Acta 359, 4441 (2006)
R. SelwinJoseyphus, M. Sivasankaran Nair, Arab. J. Chem. 3, 195 (2010)
S.B. Kalia, K. Lumba, G. Kaushal, M. Sharma, Indian J. Chem. A 46A, 1233 (2007)
L.M. Venanzi, J. Inorg. Nucl. Chem. 8, 137 (1958)
O.A.M. Ali, Spectrochim. Acta A 132, 52 (2014)
N. Raman, Y. Pitchaikani Raja, A. Kulandaisamy, Proc. Indian Acad. Sci. (Chem. Sci.) 113, 183 (2001)
K.D. Karlin, J. Zubieta (eds.), Copper Coordination Chemistry: Biochemical and Inorganic Perspectives (Adenine Press, Guilderland, NY, 1983)
A.M. Mansour, J. Therm. Anal. Calorim. 123, 571 (2016)
A. Majumder, G.M. Rosair, A. Mallick, N. Chattopadhyay, S. Mitra, Polyhedron 25, 1753 (2006)
A.W. Varnes, R.B. Dodson, E.L. Wehry, J. Am. Chem. Soc. 94, 946 (1972)
B.E. Warren, X–ray Diffraction (Dover, New York, 1990)
B. Mondak, B. Sen, S. Sarkar, E. Zangrando, P. Chattopadhyay, J. Chem. Sci. 129, 45 (2017)
S.E. Sherman, D. Gibson, A.H.J. Wang, S.J. Lippard, J. Am. Chem. Soc. 110, 7368 (1988)
L. Strekowski, B. Wilson, Mutat. Res. 623, 3 (2007)
P.K. Sasmal, A.K. Patra, A.R. Chakravarty, J. Inorg. Biochem. 102, 1463 (2008)
Acknowledgements
The authors wish to thank the Director, CMET, Hyderabad, for providing TG facility. The authors are thankful to the Department of Biochemistry, SV University, Tirupati, for providing antioxidant activity, Department of Biotechnology and University College of Pharmaceutical Sciences, Kakatiya University, Warangal, for antimicrobial and anti-proliferative activity studies. We also wish to thank the Ministry of Human Resource Development for granting the research fellowship to B. Mayuri.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bheemarasetti, M., Palakuri, K., Raj, S. et al. Novel Schiff base metal complexes: synthesis, characterization, DNA binding, DNA cleavage and molecular docking studies. J IRAN CHEM SOC 15, 1377–1389 (2018). https://doi.org/10.1007/s13738-018-1338-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-018-1338-7