Abstract
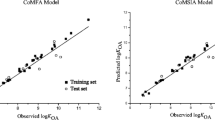
The maximum steady-state flux of 79 compounds (substituted benzenes, and quinolones and their derivatives) with a wide range of polarity through a PDMS membrane was predicted using a comparative molecular field analysis (CoMFA) and comparative molecular similarity index analysis (CoMSIA) methods. Moreover, the contribution of partial atomic charge to mass transport phenomena was further verified by the correlation of atomic charge to apparent permeability through polydimethylsiloxane (PDMS) membranes. The obtained results indicated superiority of CoMSIA model over CoMFA model. The best CoMSIA model is developed based on the combination of electrostatic and hydrophobic and H-bond acceptor fields (CoMSIA-EHA). The contour maps of electrostatic and hydrophobic and H-bond acceptor fields of CoMSIA model provide an interpretable and logical relationship between chemicals structure and their fluxes, which give useful insights for designing new compounds with higher penetration through the membranes.




Similar content being viewed by others
References
H.A. Daynes, The process of diffusion through a rubber membrane. Proc. R. Soc. Lond. A97, 286–307 (1920)
J. Crank, S. Park, Gdiffusion in Polymer (Academic Press, NY, 1968), pp. 1–39
C.S. Leopold, H.I. Maibach, J. Inv. Der. 113(3), 304–307 (1999)
A.D. Woolfson, D.F. Mccafferty, Percutaneous local anesthesia: drug release characteristics of the amethocaine phase–change systems. Int. J. Pharm. 94, 75–80 (1993)
N.A. Magrab, A.C. Williams, B.W. Barry, Estradiol permeation through human skin and statistic membrane-effects of propylene glycol and super saturation. J. Cont. Rel. 36, 277–294 (1995)
M.D. Barrate, Quantitative structure-activity relationships for permeability. Toxicol. Vitro 9, 27–37 (1995)
J.C. Dearden, Applications of quantitative structure-property relationships to pharmaceutics. Chem. Int. Lab. Syst. 24, 77–87 (1994)
D.R. Friend, In vitro skin permeation techniques. J. Controll. Rel. 18, 235–248 (1992)
E.J. Lien, H. Gao, QSAR analysis of skin permeability of various drugs in man as compared to in vivo and in vitro studies. Pharm. Res. 12, 583–587 (1995)
E.R. Garret, P.B. Chemburkar, J. Pharm. Sci. 57(6), 949–959 (1968)
D.M. Moeckly, L.E. Matheson, The development of a predictive method for the estimation of flux through polydimethylsiloxane membrane: I. Identification of critical variables for a series of substituted benzenes. Int. J. Pharm. 77, 151–162 (1991)
M.W. Hu, L.E. Matheson, The development of a predictive method for the estimation of flux through polydimethylsiloxane membrane. Ш. Application to a series of substituted pyridines. Pharm. Res. 10, 732–736 (1993)
L.E. Matheson, M.W. Hu, The development of a predictive method for the estimation of flux through polydimethylsiloxane membrane. Application to a series of substituted quinolones. Pharm. Res. 10, 839–842 (1993)
C.J. Cramer, G.R. Famini, A.H. Lowrey, use of calculated quantum chemical properties as surrogates for solvatochromic parameters in structure-activity relationship. Acc. Chem. Res. 26, 599–605 (1993)
Y. Chen, W.L. Yang, L.E. Matheson, Prediction of flux through polydimethylsiloxane membranes using atomic charge calculations. Int. J. Pharm. 94, 81–88 (1993)
R. Liu, L.E. Matheson, Comparative molecular field analysis combined with physicochemical parameters for prediction of polydimethylsiloxane membrane. Pharm. Res. 11, 257–266 (1994)
Y. Chen, P. Vayumhasuwan, L.E. Matheson, prediction of flux through polydimethylsiloxane membranes using atomic charge calculations; application to an extended data set. Int. J. Pharm. 137, 149–158 (1996)
R.D. Cramer, D.E. Patterson, J.D. Bunce, Comparative molecular field analysis (CoMFA). 1. Effect of shape on binding of steroids to carrier proteins. J. Am. Chem. Soc. 110, 5959–5967 (1988)
G. Klebe, U. Abraham, comparative molecular similarity index analysis (CoMSIA) to study hydrogen-bonding properties and to score combinatorial libraries. J. Comput. Appl. Mol. 13, 1–10 (1999)
G. Klebe, U. Abraham, T. Mietzner, molecular similarity indices in a comparative analysis (CoMSIA) of drug molecules to correlate and predict their biological activity. J. Med. Chem. 37, 4130–4146 (1994)
R. Thaimattam, P. Daga, S.A. Rajjak, R. Banerjee, J. Iqbal, 3D-QSAR CoMFA, CoMSIA studies on substituted ureas as Raf-1 kinase inhibitors and its confirmation with structure-based studies. Biorg. Med. Chem. 12, 6415–6425 (2004)
B. Wendt, R.D. Cramer, Challenging the gold standard for 3D-QSAR: template CoMFA versus X-ray alignment. J. Comput. Aided Mol. Des. 28, 803–824 (2014)
C. Xue, S. Cui, M. Liu, Z. Hu, B. Fan, 3D QSAR studies on antimalarial alkoxylated and hydroxylated chalcones by CoMFA and CoMSIA. Eur. J. Med. Chem. 39, 745–753 (2004)
W. Zhu, G. Chen, L. Hu, X. Luo, C. Gui, C. Luo, C.M. Puah, K. Chen, H. Jiang, QSAR analyses on ginkgolides and their analogues using CoMFA, CoMSIA, and HQSAR. Biorg. Med. Chem. 13, 313–322 (2005)
G. Schüürmann, R.-U. Ebert, J. Chen, B. Wang, R. Kühne, External validation and prediction employing the predictive squared correlation coefficient, Test set activity mean vs training set activity mean. J. Chem. Inf. Model. 48, 2140–2145 (2008)
B. Chen, Z. Zhu, M. Chen, W. Dong, D. Li, Three-dimensional quantitative structure–activity relationship study on antioxidant capacity of curcumin analogues. J. Mol. Struct. 1061, 134–139 (2014)
W. Samee, J. Ungwitayatorn, C. Matayatsuk, J. Pimthon, 3D-QSAR studies on phthalimide derivatives as HIV-1 reverse transcriptase inhibitors. Sci. Asia 30, 81–88 (2010)
A. Golbraikh, A. Tropsha, Beware of Q2. J. Mol. Graph. Model. 20, 269–276 (2002)
A. Tropsha, Best practices for QSAR model development, validation, and exploitation. Mol. Inform. 29, 476–488 (2010)
P.P. Roy, K. Roy, On some aspects of variable selection for partial least squares regression models. QSAR Comb. Sci. 27, 302–313 (2008)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Behgozin, S.M., Fatemi, M.H. 3D-QSAR modeling of maximum steady-state fluxes of some substituted benzenes and quinolone derivatives through polydimethylsiloxane membrane. J IRAN CHEM SOC 15, 1293–1300 (2018). https://doi.org/10.1007/s13738-018-1328-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-018-1328-9