Abstract
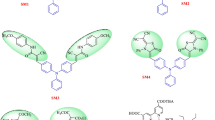
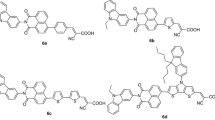
In this work, we report synthesis and device fabrication studies of four metal-free D–A-type dyes (A1–A4) based on structurally simple N,N-dimethyl-4-vinyl aniline carrying four different acceptor/anchoring groups, as sensitizers for sensitizing photoanode (TiO2). In the sensitizers, N,N-dimethylaniline ring acts as an electron donor, while barbituric acid, N,N-dimethyl barbituric acid, thiobarbituric acid and N,N-diethyl thiobarbituric acid function as electron acceptor/anchoring units. They were synthesized in good yield via Knoevenagel protocol in neutral condition without any catalyst. Further, they were subjected to structural, electrochemical and optical characterization in order to evaluate their structure, band gap and absorption/emission behavior. The studies reveal that all the four dyes have thermodynamic feasibility of electron injection as well as electron recombination; their optical band gaps were found to be in the range of 2.35–2.56 eV. High-quality crystals of A2 and A4 were grown by slow evaporation technique using its solution with 1:1 pet ether (60–80 °C)/ethyl acetate solvent mixture at room temperature. Their SC-XRD studies disclose that the crystals are in the triclinic system with space group P-1. Further, DFT studies were performed using Turbomole V7.1 software package to evaluate their optimized geometry and HOMO and LUMO levels. Finally, DSSC device fabricated with the dye A1 showed relatively good efficiency when compared to other dyes mainly due to the effective binding of barbituric acid on the surface of TiO2 through NH or OH functional group.
Graphical Abstract










Similar content being viewed by others
References
B. O’Regan, M. Grätzel, Nature 353, 737 (1991)
Y. Ooyama, Y. Harima, Eur. J. Org. Chem. 2009, 2903 (2009)
S. Mathew, A. Yella, P. Gao, R. Humphry-Baker, B.F.E. Curchod, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, M.K. Nazeeruddin, M. Grätzel, Nat. Chem. 6, 242 (2014)
A. Mishra, M.K.R. Fischer, P. Bäuerle, Angew. Chem. Int. Ed. 48, 2474 (2009)
J.N. Clifford, E. Martínez-Ferrero, A. Viterisi, E. Palomares, Chem. Soc. Rev. 40, 1635 (2011)
D.P. Hagberg, T. Marinado, K.M. Karlsson, K. Nonomura, P. Qin, G. Boschloo, T. Brinck, A. Hagfeldt, L. Sun, J. Org. Chem. 72, 9550 (2007)
M. Liang, W. Xu, F. Cai, P. Chen, B. Peng, J. Chen, Z. Li, J. Phys. Chem. C 111, 4465 (2007)
W. Xu, B. Peng, J. Chen, M. Liang, F. Cai, J. Phys. Chem. C 112, 874 (2008)
C.-H. Yang, H.-L. Chen, C.-P. Chen, S.-H. Liao, H.-A. Hsiao, Y.-Y. Chuang, H.-S. Hsu, T.-L. Wang, Y.-T. Shieh, L.-Y. Lin, Y.-C. Tsai, J. Electroanal. Chem. 631, 43 (2009)
G. Reginato, M. Calamante, A. Dessì, A. Mordini, M. Peruzzini, L. Zani, J. Organomet. Chem. 771, 117 (2014)
S. Urnikaite, M. Daskeviciene, R. Send, H. Wonneberger, A. Sackus, I. Bruder, V. Getautis, Dyes Pigm. 114, 175 (2015)
T. Michinobu, N. Satoh, J. Cai, Y. Li, L. Han, J. Mater. Chem. C 2, 3367 (2014)
M. Komatsu, J. Nakazaki, S. Uchida, T. Kubo, H. Segawa, Phys. Chem. Chem. Phys. 15, 3227 (2013)
M. Subbaiah, R. Sekar, E. Palani, A. Sambandam, Tetrahedron Lett. 54, 3132 (2013)
S. Manoharan, S. Anandan, Dyes Pigm. 105, 223 (2014)
V.P. Novikov, S. Samdal, L.V. Vilkov, Russ. J. Gen. Chem. 74, 1247 (2004)
D.D. Babu, R. Su, A. El-Shafei, A.V. Adhikari, Electrochim. Acta 10, 198 (2016)
B. Hosseinzadeh, A. Salimi Beni, A. Najafi Chermahini, R. Ghahary, A. Teimouri, Synth. Met. 209, 1 (2015)
S. Wang, S.-H. Kim, Dyes Pigm. 80, 314 (2009)
Y. Hu, Z.-C. Chen, Z.-G. Le, Q.-G. Zheng, Synth. Commun. 34, 4521 (2004)
M.K. Haldar, M.D. Scott, N. Sule, D.K. Srivastava, S. Mallik, Bioorg. Med. Chem. Lett. 18, 2373 (2008)
Z. Wu, W. Ma, S. Meng, X. Li, J. Li, Q. Zou, J. Hua, H. Tian, RSC Adv. 6, 74039 (2016)
M.C. Rezende, P. Campodonico, E. Abuin, J. Kossanyi, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 57, 1183 (2001)
C. Saravanan, S. Easwaramoorthi, L. Wang, Dalton Trans. 43, 5151 (2014)
D.D. Babu, R. Su, P. Naik, A. El-Shafei, A.V. Adhikari, Dyes Pigm. 141, 112 (2017)
P. Naik, R. Su, M.R. Elmorsy, D.D. Babu, A. El-Shafei, A.V. Adhikari, J. Photochem. Photobiol. A 345, 63 (2017)
P. Naik, M.R. Elmorsy, R. Su, D.D. Babu, A. El-Shafei, A.V. Adhikari, Sol. Energy 153, 600 (2017)
D.D. Babu, D. Elsherbiny, H. Cheema, A. El-Shafei, A.V. Adhikari, Dyes Pigm. 132, 316 (2016)
P. Qu, G.J. Meyer, Langmuir 17, 6720 (2001)
G. Oskam, B.V. Bergeron, G.J. Meyer, P.C. Searson, J. Phys. Chem. B 105, 6867 (2001)
V. Gaidelis, E. Kamarauskas, T. Malinauskas, V. Getautis, R. Send, H. Wonneberger, I. Bruder, RSC Adv. 5, 82859 (2015)
S.E. Koops, B.C. O’Regan, P.R.F. Barnes, J.R. Durrant, J. Am. Chem. Soc. 131, 4808 (2009)
A.D. Becke, Phys. Rev. A 38, 3098 (1988)
M.J.G. Peach, P. Benfield, T. Helgaker, D.J. Tozer, J. Chem. Phys. 128, 04418 (2008)
P. Dev, S. Agrawal, N.J. English, J. Phys. Chem. A 117, 2114 (2013)
D. El-Sherbiny, H. Cheema, F. El-Essawy, A. Abdel-Megied, A. El-Shafei, Dyes Pigm. 115, 81 (2015)
P.M. Sirimanne, H. Tributsch, J. Solid State Chem. 177, 1789 (2004)
L. Zhang, J.M. Cole, A.C.S. Appl, Mater. Interfaces 7, 3427 (2015)
K.R. Justin Thomas, A. Venkateswararao, C.-P. Lee, K.-C. Ho, Dyes Pigm. 123, 154 (2015)
K. Zhou, H. Fu, L. Feng, M. Cui, J. Dai, B. Liu, Chem. Commun. 51, 11665 (2015)
Acknowledgements
The authors are thankful to National Institute of Technology, Surathkal, India, for providing necessary laboratory facilities. The authors are also thankful to the Department of Textile Engineering, Chemistry and Science at North Carolina State University for the financial support.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Naik, P., Su, R., Babu, D.D. et al. Structurally simple D–A-type organic sensitizers for dye-sensitized solar cells: effect of anchoring moieties on the cell performance. J IRAN CHEM SOC 14, 2457–2466 (2017). https://doi.org/10.1007/s13738-017-1180-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-017-1180-3