Abstract
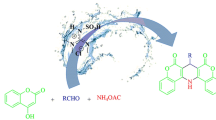
SBA-Pr-SO3H was found as a nanocatalyst for the cyclocondensation of isatoic anhydride, aromatic aldehydes and γ-amino acids (gabapentin and baclofen) to access novel 2-aryl-4-quinazolinone γ-amino acid derivatives in solvent-free reaction conditions in good to high yields. Compared to the classical approaches, this process is a simple, highly yielding, time saving, and environmentally friendly method.
Graphical abstract







Similar content being viewed by others
References
L.F. Tietze (ed.), Domino Reactions (Wiley, Weinheim, 2014)
R.P. Herera, E. Marquez-Lopéz (eds.), Multicomponent Reactions, Concepts and Applications for Design and Synthesis (Wiley, New Jersey, 2015)
R.V. Grieken, J.A. Melero, G. Morales, J. Mol. Catal. A Chem. 256, 29 (2006)
S.L. Burkett, S.D. Sims, S. Mann, Chem. Commun. 1367 (1996)
D.J. Macquarrie, Chem. Commun. 16, 1961 (1996)
X. Feng, G.E. Fryxell, L.Q. Wang, A.Y. Kim, J. Liu, K.M. Kemner, Science 276, 923 (1997)
W.M. Van Rhijn, D.E. De Vos, B.F. Sels, W.D. Bossaert, P.A. Jacobs, Chem. Commun. 317 (1998)
B. Karimi, D. Zareyee, Org. Lett. 10, 3989 (2008)
B. Karimi, A. Zamani, Org. Biomol. Chem. 10, 4531 (2012)
R.I. Kureshy, I. Ahmad, K. Pathak, N.H. Khan, S.H.R. Abdi, R.V. Jasra, Catal. Commun. 10, 572 (2009)
V. Fathi Vavsari, G. Mohammadi Ziarani, A. Badiei, S. Balalaie, J. Iran. Chem. Soc. 13, 1037 (2016)
D.J. Connolly, D.O. Cusack, T.P. Sullivan, P.J. Guiry, Tetrahedron 61, 10153 (2005)
I. Khan, A. Ibrar, W. Ahmed, A. Saeed, Eur. J. Med. Chem. 90, 12 (2015)
J. Bilbro, M. Mart, N. Kyprianou, Anticancer Res. 33, 4695 (2013)
J. Cai, M. Sun, X. Wu, J. Chen, P. Wang, X. Zong, M. Ji, Eur. J. Med. Chem. 63, 702 (2013)
A. Baba, N. Kawamura, H. Makino, Y. Ohta, S. Taketomi, T. Sohda, J. Med. Chem. 39, 5176 (1996)
Y.N. Mabkhot, M.S. Al-Har, D.A. Aldalbahi, Z. Ul-Haq, Molecules 19, 8725 (2014)
V. Alagarsamy, S.V. Raja, K. Dhanabal, Bioorg. Med. Chem. 15, 3457 (2007)
V. Alagarsamy, V.R. Solomon, K. Dhanapala, Bioorg. Med. Chem. 15, 235 (2007)
V. Jatav, S. Kashaw, P. Mishra, Med. Chem. Res. 17, 205 (2008)
M.S. Malamas, J. Millen, J. Med. Chem. 34, 1492 (1991)
ShN Khattab, N.S. Haiba, A.M. Asal, A.A. Bekhit, A. Amer, H.L. Abdel-Rahman, A. El-Faham, Bioorg. Med. Chem. 23, 3574 (2015)
M. Dabiri, P. Salehi, S. Otokesh, M. Baghbanzadeh, G. Kozehgary, A.A. Mohammadi, Tetrahedron Lett. 46, 6123 (2005)
S. Rostamizadeh, A.M. Amani, G.H. Mahdavinia, H. Sepehrian, S. Ebrahimi, Synthesis 1356 (2010)
L.M. Wang, I. Hu, J.H. Shao, J.J. Yu, L. Zhang, J. Flourine Chem. 129, 1139 (2008)
H.R. Shaterian, A.R. Oveisi, Chin. J. Chem. 27, 2418 (2009)
C. Jiuxi, W. Dengze, H. Fei, L. Miaochang, W. Huayue, D. Jinchang, S. Weike, Tetrahedron Lett. 49, 3814 (2008)
K. Ramesh, K. Karnakar, G. Satish, K.V.R. Harsha, Y.V.D. Nageswar, Tetrahedron Lett. 53, 6095 (2012)
Y. Chen, W. Shan, M. Lei, L. Hu, Tetrahedron Lett. 53, 5923 (2012)
M. Prakash, V. Kesavan, Org. Lett. 14, 1896 (2012)
M. Wang, T.T. Zhang, Y. Liang, J.J. Gao, Monatsh. Chem. 143, 835 (2012)
A. Saffar-Teluri, S. Bolouk, Monatsh. Chem. 141, 1113 (2010)
W. Shu-Liang, Y. Ke, W. Xiang-Shan, Chin. J. Org. Chem. 31, 1235 (2011)
G. Wheeler, Curr. Opin. Investig. Drugs 3, 470 (2002)
H. Stefan, T.J. Feuerstein, Pharmacol. Ther. 113, 165 (2007)
P.G. Vasudev, S. Chatterjee, N. Shamala, P. Balaram, Acc. Chem. Res. 42, 1628 (2009)
A. Salimbeni, F. Paleari, R. Canevotti, M. Criscuoli, A. Lippi, M. Angiolini, L. Belvisi, C. Scolastico, L. Colombo, Bioorg. Med. Chem. Lett. 7, 2205 (1997)
M. Tajbakhsh, S. Ramezanpour, S. Balalaie, H.R. Bijanzadeh, J. Heterocycl. Chem. 52, 1559 (2015)
E. Ghabraie, S. Mehrparvar, S. Balalaie, F. Rominger, J. Org. Chem. 79, 7926 (2014)
E. Ghabraie, S. Balalaie, Helv. Chim. Acta 97, 1555 (2014)
H.R. Bijanzadeh, S. Mehrparvar, S. Balalaie, J. Iran. Chem. Soc. 10, 1859 (2015)
S. Balalaie, M. Kassaee, H.R. Bijanzadeh, F. Darvish, M. Bararjanian, F. Jalaiyan, J. Iran. Chem. Soc. 111, 1483 (2014)
V. Fathi Vavsari, V. Dianati, S. Ramezanpour, S. Balalaie, Synlett 26, 14 (2015)
V. Fathi Vavsari, G. Mohammadi Ziarani, S. Balalaie, A. Latifi, M. Karimi, A. Badiei, Tetrahedron 72, 35 (2016)
Acknowledgment
Saeed Balalaie gratefully acknowledges Iran National Science Foundation (INSF) for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hekmat, S., Balalaie, S., Ramezanpour, S. et al. SBA-Pr-SO3H: an efficient catalyst for the combinatorial synthesis of functionalized 2-aryl-4-quinazolinones using unusual γ-amino acids. J IRAN CHEM SOC 14, 833–841 (2017). https://doi.org/10.1007/s13738-016-1033-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-016-1033-5