Abstract
A study of copper-mediated C–heteroatom especially C–N and C–O bond formations using simpler methodologies has been carried out. In the present work, acetylation of various substrates such as amines, phenols and alcohols; synthesis of 2,4,5-trisubstituted imidazole is done using simple and easily available starting materials. Copper (I) oxide was synthesized in situ by the reduction of Fehling’s solution with glucose followed by its anchoring onto different supports like silica, HAP, basic alumina and cellulose. Comparison and contrasts between the reactivity of copper (I) oxide supported onto different supports for these reactions are made. The reactivity of copper (I) oxide seems to be largely dependent on the nature of support and the most active catalyst for a particular reaction was further characterized by different spectroscopic techniques such as FTIR, XRD, TGA, XPS, SEM, TEM and AAS. The catalysts were found to be stable, easily recyclable without any significant loss in activity.
Graphical abstract
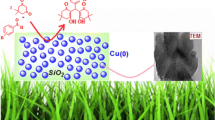
Applications of solid supported copper (I) oxides (where solid support is silica, HAP, cellulose and basic alumina) are studied for various organic transformations with special emphasis on C–N and C–O bond formation reactions.













Similar content being viewed by others
References
J.F. Hartwig, Nature 455, 314 (2008)
G.A. Artamkina, M.V. Ermolina, I.P. Beletskaya, Mendeleev Commun. 13, 158 (2003)
J.F. Hartwig, Synlett. 1997, 329 (1997)
J.F. Hartwig, Acc. Chem. Res. 31, 852 (1998)
C.G. Frost, P. Mendonca, J. Chem. Soc. Perkin Trans. 1, 2615 (1998)
A.J. Belfield, G.R. Brown, A.J. Foubister, Tetrahedron 55, 11399 (1999)
D. Prim, J.M. Campagne, D. Joseph, B. Andrioletti, Tetrahedron 58, 2041 (2002)
J.P. Wolfe, S. Wagaw, J.-F. Marcoux, S.L. Buchwald, Acc. Chem. Res. 31, 805 (1998)
J.F. Hartwig, Angew. Chem. Int. Ed. Engl. 37, 2046 (1998)
B.H. Yang, S.L. Buchwald, J. Organomet. Chem. 576, 125 (1999)
Y.R. de Miguel, E. Brule, R.G. Margue, J. Chem. Soc. Perkin Trans. 1, 3085 (2001)
B.A. Lorsbach, M.J. Kurth, Chem. Rev. 99, 1549 (1999)
C.A. Parrish, S.L. Buchwald, J. Org. Chem. 66, 3820 (2001)
M. Gupta, S. Paul, R. Gupta, Chin. J. Catal. 35, 444 (2014)
H.M. Wei, H.B. Gong, L. Chen, M. Zi, B.Q. Cao, J. Phys. Chem. C 116, 10510 (2012)
I. Baker, R.S. Gibbs, Ind. Eng. Chem. Anal. Ed. 15, 505 (1943)
W. Sun, W. Sun, Y. Zhao, Y. Chu, J. Solid, State. Chem. 184, 1638 (2011)
C.H. Kuo, M.H. Huang, Nano. Today 5, 106 (2010)
Y.X. Wang, X.F. Tang, Z.G. Yang, Colloids Surf. A Physicochem. Eng. Asp. 388, 38 (2011)
J.H. Lee, S.K. Hong, W.B. Ko, J. Ind. Eng. Chem. 16, 564 (2010)
H.L. Ruiz, J.E.C. de la Pedro, S.R. Lima, I.P. Perez, B.V.R. Sanchez, R. Santillan, O. Cereno, ARKIVOC iii, 139 (2013)
V. Beneteau, A. Olmos, T. Boningari, J. Sommer, P. Pale, Tetrahedron Lett. 51, 3673 (2010)
X.Y. Yan, X.L. Tong, Y.F. Zhang, X.D. Han, Y.Y. Wang, G.Q. Jin, Y. Qin, X.Y. Guo, Chem. Commun. 48, 1892 (2012)
K.V.R. Chary, G.V. Sagar, D. Naresh, K.K. Seela, B. Sridhar, J. Phys. Chem. B. 109, 9437 (2005)
A.R. Hajipour, F. Mohammadsaleh, J. Iran. Chem. Soc. (2015). doi:10.1007/s13738-015-0599-7
S.H. Tohidi, Int. J. Nanosci. Nanotechnol. 7, 7 (2011)
Y. Qian, F. Ye, J. Xu, Z.G. Le, Int. J. Electrochem. Sci. 7, 10063 (2012)
T.W. Greene, P.G.M. Wuts, Protective Groups in Organic Synthesis, 3rd edn. (Wiley, New York, 1999)
P.J. Kocieński, Protecting Groups (Georg Thieme Verlag, New York, 1994)
S. Naik, G. Bhattacharjya, V.R. Kavala, B.K. Patel, ARKIVOC i, 55 (2004)
P. Phukan, Tetrahedron Lett. 45, 4785 (2004)
R.H. Baker, F.G. Bordwell, Organic Synthesis Collective, vol. III (Wiley, New York, 1995), p. 141
S.V. Pansare, M.G. Malusare, A.N. Rai, Synth. Commun. 30, 2587 (2000)
I.M. Baltork, H. Aliyan, A.R. Khosropour, Tetrahedron 57, 5851 (2001)
S.K. De, Tetrahedron Lett. 45, 2919 (2004)
A.K. Chakraborti, R. Gulhane, Tetrahedron Lett. 44, 6749 (2003)
R. Dalpozzo, A.D. Nino, L. Maiuolo, A. Procopiou, M. Nardi, G. Bartoli, R. Romeo, Tetrahedron Lett. 44, 5621 (2003)
M.L. Kantam, K. Aziz, P.R. Likhar, Catal. Commun. 7, 484 (2006)
R. Ghosh, S. Maiti, A.K. Chakraborty, Tetrahedron Lett. 46, 147 (2005)
A. Kamal, M. Naseer, A. Khan, K.S. Reddy, Y.V.V. Srikanth, T. Krishnaji, Tetrahedron Lett. 48, 3813 (2007)
B. Das, P. Thirupathi, J. Mol. Catal. A: Chem. 269, 12 (2007)
T.S. Reddy, M. Narasimhulu, N. Suryakiran, K.C. Mahesh, K. Ashalatha, Y. Venkateswarlu, Tetrahedron Lett. 47, 6825 (2006)
J.S. Yadav, A.V. Narsaiah, A.K. Basak, P.R. Goud, D. Sreenu, K. Nagaiah, J. Mol. Catal. A: Chem. 255, 78 (2006)
J.K. Joseph, S.L. Jain, B. Sain, J. Mol. Catal. A: Chem. 267, 108 (2007)
K. Shyamprasad, S.Z.M. Shamshuddin, V.T. Vasantha, J. Porous Mater. 21, 1079 (2014)
S. Farhadi, K. Jahanara, A. Sepahdar, J. Iran. Chem. Soc. 11, 1103 (2014)
F. Dehghani, A.R. Sardarian, M.M. Doroodmand, J. Iran. Chem. Soc. 11, 673 (2014)
S. Farhadi, K. Jahanara, Chin. J. Catal. 35, 368 (2014)
T.E. Kristensen, Beilstein J. Org. Chem. 11, 446 (2015)
R.K. Sodhi, V. Kumar, S. Paul, Open Catalysis J. 6, 1 (2013)
A.A. Marzouk, V.M. Abbasov, A.H. Talybov, Chem. J. 2, 179 (2012)
S.V. Nalage, M.B. Kalyankar, V.S. Patil, S.V. Bhosale, S.U. Deshmukh, R.P. Pawar, Open Catalysis J. 3, 58 (2010)
M. Gupta, M. Gupta, S. Paul, V.K. Gupta, R. Khajuria, Monatsh. Chem. 146, 143 (2015)
G.J. Poinem, R. Brundavanam, X.T. Le, S. Diordjevic, M. Prokic, D. Fawcett, Int. J. Nanomed. 6, 2083 (2011)
A. Chandrasekar, S. Sagadevan, A. Dakshnamoorthy, Int. J. Phys. Sci. 8, 1639 (2013)
A. Singh, K.M. Purohit, J. Biotechnol. Biomater. 2, 104 (2011)
S. Paul, P. Nanda, R. Gupta, A. Loupy, Tetrahedron Lett. 43, 4261 (2002)
B.S. Furniss, A.J. Hannaford, P.W.G. Smith, A.R. Tatchell, Vogel’s Textbook of Practical Organic Chemistry, 5th edn. (Longman-ELBS, London, 1991), p. 1370
Handbook of Fine Chemicals, Sigma Aldrich (Advancing Science), vol. 2007–2008, p. 629
G. Brahmachari, S. Laskar, S. Sarkar, Indian J. Chem. 49B, 1274 (2010)
Research Chemicals, Metals and Materials, Alfa aesar (A Johnson Matthey Company), vol. 2011–2013, p. 1124
R. Gupta, V. Kumar, M. Gupta, S. Paul, R. Gupta, Indian J. Chem. 47B, 1739 (2008)
L.R. Steffel, T.J. Cashman, M.H. Reutershanand, B.R. Linton, J. Am. Chem. Soc. 129, 12956 (2007)
S. Farhadi, S. Panahandehjoo, Eur. J. Chem. 1, 335 (2010)
T.S. Jin, Y.R. Ma, T.S. Li, Z.H. Zhang, G.B. Duan, Indian J. Chem. 38B, 109 (1999)
M.M. Mojtahedi, M.S. Abaee, M. Javadpour, Phosphorus Sulfur Silicon Relat. Elem. 185, 2362 (2010)
J. Safari, S.D. Khalili, S.H. Banitaba, J. Chem. Sci. 122, 437 (2010)
M.M. Heravi, M. Zakeri, H. Haghi, Synth. React. Inorg. Metal-Org. Nano-Metal Chem. 41, 1310 (2011)
Acknowledgments
We are grateful to Director, SAIF, Punjab University, Chandigarh, for TEM and XRD and also to Head, SAIF, IIT Bombay, for recording SEM images. We extend our sincere thanks to UGC, New Delhi, for financial support to purchase FTIR; awarding Major Research Project F 41‐281/2012 (SR), and Prof. R.K. Bamezai, Department of Chemistry, University of Jammu, for recording TGA.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
13738_2015_730_MOESM2_ESM.doc
Supplementary material 2 (DOC 3046 kb) Supplementary information 1: 1H NMR and 13C NMR of some selected N-acetylated, O-acetylated products and 2,4,5-trisubstituted imidazoles
Rights and permissions
About this article
Cite this article
Gupta, M., Gupta, M. Doping of copper (I) oxide onto a solid support as a recyclable catalyst for acetylation of amines/alcohols/phenols and synthesis of trisubstituted imidazole. J IRAN CHEM SOC 13, 231–241 (2016). https://doi.org/10.1007/s13738-015-0730-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-015-0730-9