Abstract
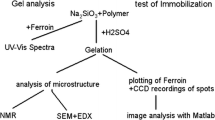
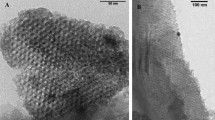
Transparent sol–gel thin films immobilized with 1,10-phenanthroline were prepared via the acid-catalyzed sol–gel reaction of tetraethylorthosilicate (TEOS) in the presence of phen. Three surfactants that include cationic cetyl trimethyl ammonium bromide (CTAB), anionic sodium dodecyl sulfate (SDS) and non-ionic Triton X-100 (TX-100) were tested for the improvement of the host material mesostructure, increasing its porosity and good accommodation of the phen within the silica matrix. The immobilized phen thin films showed similar behavior as their free counterpart in aqueous solution. The immobilized phen complex retained its activity toward Fe2+ metal ions by immobilization and produced a stable complex without any shift in the wavelength of Vis absorption spectra comparing with its free counterpart. Different parameters including concentrations of phen, Fe2+ and surfactant, type of surfactant, lifetime and number of measurements were investigated. The phen thin film sensor showed high sensitivity, chemical stability, good reproducibility and long lifetime behavior. The immobilized phen thin films were used for quantification of iron(II) in versatile aqueous samples. The polarized light microscopy indicated that the phen molecules were distributed uniformly within the host silica network.


















Similar content being viewed by others
References
W. Jin, J.D. Brennan, Anal. Chim. Acta 461, 1 (2002)
C.J. Brinker, G.W. Scherer, Sol–Gel Science (Academic Press, New York, 1990)
J. Livage, T. Coradin, C. Roux, J. Phys. Condens. Matter 13, R673 (2001)
I. Gill, A. Ballesteros, Trends Biotechnol. 18, 282 (2000)
S. Sakka, Handbook of Sol-Gel Science and Technology Processing, Characterization and Applications (Kluwer, USA, 2005)
S.J. Darzi, J. Iran. Cem. Soc. 11(5), 1363 (2014)
X.H. Chen, Y.B. Hu, G.S. Wilson, Biosens. Bioelectron. 17, 1005 (2002)
R. Zusman, C. Rottman, M. Ottolenghi, D. Avnir, J. Non-Cryst. Solids 122, 107 (1990)
L.L. Hench, I.K. West, Chem. Rev. 90, 33 (1990)
L.C. Klein, B. Dun, In: Sol-Gel Technology, (eds) (Noyes Publications, Park Ridge, NJ, 1988)
H. Boettcher, J. Prakt. Chem. 342 (2000)
J.D. Wright, N.A.J.M. Sommerdijk, Sol-Gel Materials: Chemistry and Applications (Gordon and Breach Science Publishers, Amsterdam, 2001)
J. Samuel, A. Strinkoviki, S. Shalom, K. Lieberman, M. Ottolenghi, D. Avnir, A. Lewis, Mater. Lett. 21, 431 (1994)
N.M. El-Ashgar, A.I. El-Basioni, I.M. El-Nahhal, S.M. Zourob, T.M. El-Agez, S.A. Taya, ISRN Anal. Chem. (2012). doi:10.5402/2012/604389
M.J.P. Leiner, O.S. Wolfbeisin, O.S. Wolfbeis, In: Fiber Optic Chemical Sensors and Biochemical Sensors, (eds) (CRC Press, Boca Raton, 1991), pp. 359
O.S. Wolfbeis, N.V.T. Rodriguez, Werner Mikrokim Acta 108, 133 (1992)
L.A. Saari, W.R. Seitz, Anal. Chem. 54, 821 (1982)
L. Lobnik, I. Oehme, I. Murkoic, O.S. Wolfbeis, Anal. Chim. Acta 367, 159 (1998)
L.M. Ellerby, C.R. Nishida, F. Nishida, S.A. Yamanakas, B. Dunn, S.J. Valentine, J.I. Zink, Science 255, 1113 (1992)
G. Wirnsberger, P. Yang, B.J. Scott, B.F. Chemelka, G. Stuky, Spectrochim. Acta, Part A 57, 2049 (2001)
I.M. El-Nahhal, S.M. Zourab, F.S. Kodeh, A. Al-Bawab, Int. J. Anal. Chem. 90, 644 (2010)
G.G. Guilbality, Analytical Uses of Immobilized Enzymes (Marcel Dekker, New York, 1989), p. 77
N.D. Irhand, T. Elmali, Nebahat, Turk. J. Chem. 27 (2003)
M. Pagliaro, R. Ciriminna, G. Palmisano, Chem. Soc. Rev. 36 (2007)
N.M. Yoshioka, H. Inoue, Transit. Met. Chem. 24 (1999)
R. John, Healthy, The Scientifically Proven Secrets of the World’s Healthiest and Longest-Lived Peoples, 1st edn. (Ballantine Books, USA, 2007)
WHO, Guidelines for Drinking-Water Quality, 2nd edn. vol 2. Health Criteria and Other Supporting Information (World Health Organization, Geneva, 1996)
O. Lev, Analusis 20, 543 (1992)
M.J. Paterson, B. Ben-Nissan, Surf. Coat. Technol. 86(1), 153 (1996)
Acknowledgments
The authors thank the Islamic University of Gaza for financial support and Al-Azhar University of Gaza for technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Ashgar, N.M., Bolbol, H.Z. Sol–gel derived silica thin films immobilized with 1,10-phenanthroline and their binding activity toward iron(II). J IRAN CHEM SOC 12, 791–799 (2015). https://doi.org/10.1007/s13738-014-0540-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-014-0540-5